
Real Time Release Testing

Real Time Release Testing

Chemistry reviewers in FDA's Office of Generic Drugs provide an overview of common deficiencies cited throughout the CMC section of ANDAs.

Deep process characterization and "lab-on-a-chip" enable SMART bioprocess design.

Follow-ons were all the rage at this year's JP Morgan Healthcare Conference.

Industry and regulatory experts discuss the challenges and benefits of implementing real time release testing in a pharmaceutical manufacturing environment.

Government and private sector efforts take on counterfeit drugs online.
Will 2011 be a more promising year for new molecular entities? A review of Big Pharma's late-stage pipeline shows what might lie ahead.

New products in tableting and granulation.

As biologic-drug patents move toward expiration in the US, Indian firms with experience in the follow-on biologics arena are eager to partner with global manufacturers and secure their place in the growing biosimilars market.

Legislation has hampered cross-border drug importation and limited choice.

Q&A with Magnetrol International's Dan Klees

Food-safety, transparency, and counterfeit-drug growth will tax agency resources.
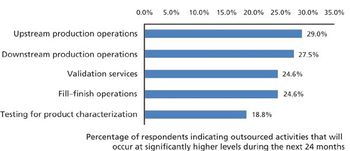
More crucial biomanufacturing operations are expected to be outsourced.

Anticounterfeiting solutions for vials and syringes.

USP helps to improve drug quality in 32 countries.

Taking care to note, file and re-check information can save one from future mishaps.

Using risk assessment properly can provide industry with a unique tool for quality control.

Nearly six years after applying, the FDA joins the Pharma Inspection Co-operation Scheme.

How to adapt a real time release approach to powder processing during drug-product manufacturing.

The author describes why statistical significance would impose an unreasonable burden on manufacturers.
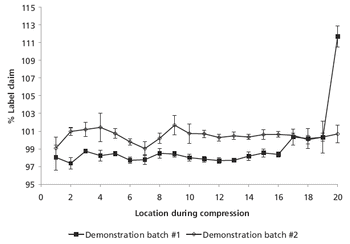
The authors modified equipment and the manufacturing process to re-establish content uniformity among tablets.

Innovations protect the quality of temperature-sensitive products.

A roundtable moderated by Angie Drakulich.