
PharmTech speaks to Chris Eccles, managing director of ChargePoint Technology.

PharmTech speaks to Chris Eccles, managing director of ChargePoint Technology.

Medication safety and efficacy depend on maintaining products at the proper temperature.

Siegfried Schmitt, a principal consultant with PAREXEL, discusses how the EU's Falsified Medicines Directive will affect US API production.

Is the contract-only CMO an endangered species?

Why social media presents unique challenges and opportunities for pharmaceutical companies.
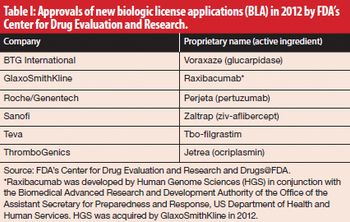
The share of biologic-based drugs in the global pharmaceutical market is on the rise.

Quality assurance of biological products is central to India's good distribution practices guidelines.

Measuring the rouge corrosion rate can help determine when a system should be cleaned so the final product is not impacted

A round up of news from across the web, including trends from social social media platforms.

Prosecutors and regulators challenge manufacturing quality failings likely to cause patient harm.
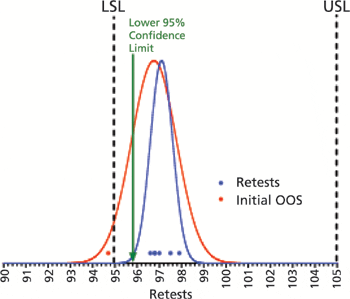
Two methods to evaluate retest data following out-of-specification results.

A thorough investigation of all possible causes of deviations should be performed.

Eastern Europe is moving towards a goal of harmonized regulations.

The minimum amount of residue that can be visually detected is demonstrated for a small number of active pharmaceutical ingredients (APIs) on a range of different surface materials.

The US Pharmacopeial Convention contiues to make modernization of standards a top priority in 2013.

A roundup of regulatory news from across the global pharmaceutical industry.

New product reviews for February 2013.
Adopting a seven-step process to maintenance and storage improves tableting quality.

Protecting patients from counterfeit medicines is a pressing issue facing governments and the pharmaceutical industry.

Even in an industry in which all product development is complicated by the intricacies of human biology, orally inhaled products (OIP) stand out as singularly demanding.
Researchers use inorganic catalysts as an alternative to biocatalysts in the selective conversion of sugars to produce chiral building blocks.

Recent activity in standards-setting organizations has raised interest in the impact of testing for impurities that may enter the product before it is mined or harvested or even due to intentional use of some reagents.

Seeking Cold-Chain Efficiency