
Integrated data and cloud-based solutions can be used for process optimization.

Integrated data and cloud-based solutions can be used for process optimization.
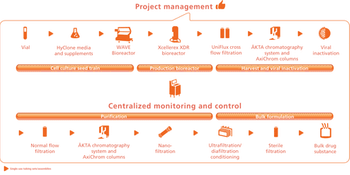
Asking the right questions is crucial to establishing a biopharmaceutical facility design.

Policy makers debate strategies for promoting access to less costly medicines.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how the regulatory requirements for CGMPs is the different phases of drug development and manufacture.

Data integrity is a widespread, global problem that must be addressed.

Slides from the FDA GDUFA presentation before the Senate Health, Education, Labor and Pensions Committee in January 2016.

Thought leaders tackle drug shortages and manufacturing innovations.

Gauging the adequate level and type of screening is the challenge when identifying relevant crystalline forms.

Standardized testing protocols are crucial for acceptance of single-use systems.

The Spark 20M multimode microplate reader has the ability to read 6- to 1536-well microplates and includes a high-frequency xenon flash lamp that can be combined with detection modules.

The Dionex Integrion High-Pressure Ion Chromatography system from Thermo Scientific includes high-pressure capability and electrochemical detection.

The Zeiss Smartproof 5 wide-field confocal microscope is designed for applications in quality assurance and quality control, production, and R&D laboratories.

The pharma outsourcing market starts 2016 with expansions, acquisitions, and new offerings.

Supply-chain success is measured by how effectively new medications reach patients, and how swiftly manufacturers can react to internal and external changes.

The EU is striving to reduce the costs and administrative burden facing pharmaceutical manufacturers when complying with variations regulations for keeping authorization dossiers up to date.

Choosing the right facility size requires tailoring the design for current needs as well as anticipating the future.

There are many different approaches for assessing process parameter criticality, and assessing which process parameters have a significant impact on critical quality attributes (CQAs) is a particular challenge. Including an unimportant process parameter as a critical process parameter (CPP) in a control strategy can be detrimental. The authors present a statistical approach to determine when a statistically significant relationship between a process parameter and a CQA is large enough to make a practically meaningful impact (i.e., practical significance).

As specialty API outsourcing grows, manufacturers and contract development and manufacturing organizations are investing for the long haul.

Data integrity has become a more serious compliance problem at pharmaceutical manufacturing plants throughout the world.

The IH-TMS Tool Management System from I Holland monitors tool rotations, tooling inventory, and maintenance.

High Speed Dispersers from Ross feature a National Electrical Manufacturers Association (NEMA) 7&9 Operator Panel and grounding systems.


The Class 100 cleanroom oven from Grieve is used for a variety of heat processes such as sterilizing, depyrogenation, curing, and drying workloads.

Click the title above to open the Pharmaceutical Technology March 2016 issue in an interactive PDF format.