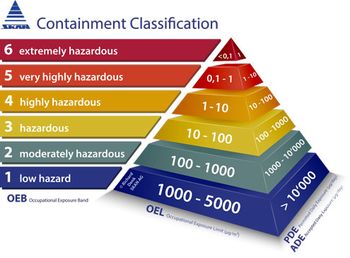
The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

Policies limiting imports and immigration generate uncertainty for US and foreign firms

Effective solutions for overcoming the high molecular weight, hydrophilicity, and instability of large biomolecules have yet to be identified.

Siegfried Schmitt, PhD, Principal Consultant at PAREXEL, discusses how to mitigate risk in a global regulatory environment.

A survey on risk-based predictive stability tools reveals how pharma companies are leveraging advanced stability approaches throughout the drug development process.

A robust quality agreement and good communication scheme can help avoid and alleviate regulatory concerns.

Moving to the next level of productive, reliable performance in bio/pharmaceutical manufacturing requires a willingness to make changes and create a quality culture.
The DME Facility Focus survey revealed best practices for coping with the challenges of aging facilities and implementing facility modernization.

The OCEASOFT Loading Bench is an innovation for cold-chain monitoring management for transportation and high-volume shipment.

The Pharmafill TC4 Tabletop electronic tablet counter from Deitz Co. automatically counts pills, tablets, capsules, softgels, caplets, lozenges, and other solid oral-dose products and fills them into bottles.

Ross, Charles & Son offers a variety of mixers for mixing and blending applications.

The Rainin SmartStand from Mettler Toledo includes four pipette holders.

Industry experts discuss IIoT and its impact on pharmaceutical manufacturing.

Drug type, potential sales, and ownership factor in the race to get drugs to market.

Quality metrics are used by FDA and by bio/pharma companies to evaluate manufacturing and fuel continuous improvement efforts.

Pharmaceutical Technology spoke with Frank Generotzky, plant manager for Baxter BioPharma Solutions’ Halle, Germany facility, about operational excellence at the site.
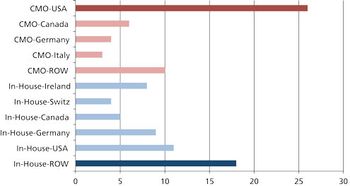
Moving global manufacturing operations may be more complicated than it appears.

Pharmaceutical Technology asked Siegfried Schmitt, principal consultant at PAREXEL, about the importance of quality agreements in the sponsor/contractor relationship.

This study investigated the stability of solid lactose stored under high temperature and humidity conditions.

The authors discuss the challenges of capsule filling in preclinical and clinical studies.

Capsule filling is a complex process, and the product to be encapsulated must be well developed to ensure mass uniformity.

Although best practices are key, advances in integrated informatics platforms and automation can make it easier to ensure data integrity and improve overall lab efficiency.

As the November 2017 deadline nears, a surprising number of companies still don’t have a serialization plan in place. New programs aim to get them compliant in time.

The author discusses the results from TraceLink and Actionable Research's Global Drug Supply, Safety and Traceability Report.

Johnson & Johnson Supply Chain (JJSC) and the distributor AmerisourceBergen launched a four-week pilot program to test GS1’s EPCIS standards and to see how effectively data could be transferred between the two partners.

Click the title above to open the Pharmaceutical Technology March 2017 issue in an interactive PDF format.