
Honeywell has developed a cloud-based simulation tool that uses a combination of augmented reality and virtual reality to train plant personnel.

Honeywell has developed a cloud-based simulation tool that uses a combination of augmented reality and virtual reality to train plant personnel.

Ross, Charles & Son has added a 500-gallon option to its line of VersaMix multi-shaft mixers.

The upcoming serialization deadline and the United Kingdom's departure from the European Union could result in supply bottlenecks.

This article describes a new, combined, quantitative method for assessing excipient risks that has been developed by the authors as one possible risk evaluation method.
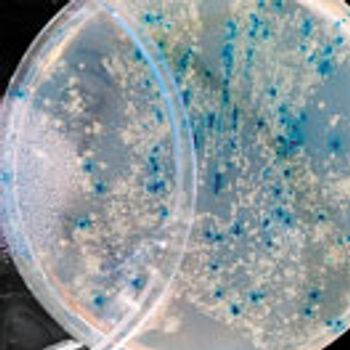
Case studies compare efficacy testing of preservatives for topical formulations with probiotic actives.

Manufacturers face demands for timely information on clinical studies, product recalls, and approvals.
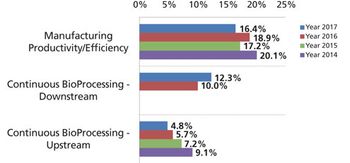
Development and adoption of new technologies create challenges that may take years to resolve.

Endress+Hauser's Memosens COS81D hygienic optical sensor can measure dissolved oxygen in pharmaceutical fermenters and bioreactors.

The ZSE 50 MAXX twin screw extruder system from Leistritz Extrusion is suited for a range of compounding tasks.

In-house experts can help select the right systems and suppliers, making validation and compliance easy, says Siegfried Schmitt, principal consultant at PAREXEL.

New and emerging products advance bio/pharma laboratory operations.

FDA enforcement efforts and drug approvals trend upward.

Assessing potential formulation and manufacturing issues in early development phases can improve a drug’s chances for success.

Drug companies must consider all factors impacting the cost of drugs, including high costs of early development stages.

Alan Kennedy, executive director of TEAM UP, shared perspectives on Poseidon and ocean transport with Pharmaceutical Technology.

Lower costs, fewer opportunities for temperature excursions, and a smaller carbon footprint are making ocean transport more attractive for pharmaceuticals. Poseidon, a new collaborative pharma initiative, seeks to leverage benefits.

Aston Particle Technologies has developed a technology that produces functionalized particles in a one-step, environmentally friendly process.

Robert Iser of PAREXEL Consulting answers questions regarding the regulatory expectations of quality agreements and how companies can ensure the quality and safety of their products.

Stability testing on APIs/finished drug product helps define optimal drug packaging for shelf-life storage.

Highly complex APIs developed to treat rare and orphan diseases present big technical questions for contract developers.

Click the title above to open the Pharmaceutical Technology March 2018 issue in an interactive PDF format.

Drug manufacturers can improve use of quality agreements in contract manufacturing.