
Thinking Inside the Box

Thinking Inside the Box

Defining the Next Five Years.

Manufacturers will pay new fees but anticipate expanded drug use and safeguards for innovation.

A method for determining sample size is finally getting some respect.

Lorensen discusses client demands and industry trends.

FDA wants industry to talk to them about the science underlying process innovations-really.
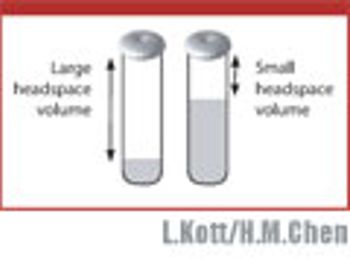
In this case study of amines, the authors discuss several parameters to be considered in developing a headspace GC method.

Eli Lilly faces up to the new realities of the bio/pharmaceutical industry through outsourcing.

Chemocatalysis and biocatalysis are important elements of an effective strategy for improving yield and stereoselectivty.

The authors demonstrate that sustained-release delivery can help avoid the risk of sudden higher-blood concentration of a drug to avoid toxicity.

The growth of Brazil's generic-drug market is on a fast track, but what are the projections for the sector's future?

A book helps statistics novices prepare to comply with the US Food and Drug Administration's draft guidance on process validation.

A Pharma Business Imperative.

Editors' Picks of pharmaceutical science and technology innovations

Agreement on standards for excipient qualification, development, and fair pricing is underway.

A modular approach to biopharmaceutical production could bring process flexibility, and contract manufacturing organizations are beginning to take notice.

When vessels, seals, and cooling units go haywire, operators must get in the mix.