
Dolomite Microfluidics has developed the Drug Encapsulation System to provide scientists with a simple and scalable way of encapsulating drug samples.

Dolomite Microfluidics has developed the Drug Encapsulation System to provide scientists with a simple and scalable way of encapsulating drug samples.
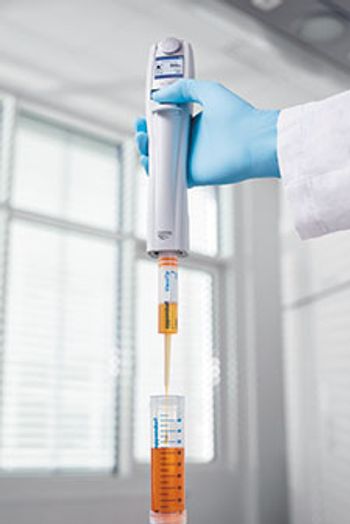
Eppendorf’s ViscoTip pipette tip from its Combitips advanced line can process liquids with viscosities from 200 to 14,000 mPa-s, including glycerol 99.5%, ointments, and creams.

Ross, Charles & Son's SysCon’s programmable logic controller (PLC) system features a control panel with a wireless connection that allows users to access the PLC system using tablets, laptops, and smartphones via Ethernet, WLAN, or Bluetooth.

The MagMixer MBE Series from SPX Flow is suitable for low-viscosity blending, dissolving solids, and solid suspension in sterile applications.

Choice of carrier can have a significant impact on the capsule filling process as well as the performance of the DPI formulation.

Determining the right process conditions for a freeze-drying cycle requires an understanding of the effect of each step on the drug product.

The European Union is collaborating with the Pharmaceutical Inspection Co-operation Scheme to develop similar guides for evaluating inspectorates’ competency.

Updated guidelines and new technologies aid visual inspection of parenteral products and packaging.

Airflow visualization studies, or smoke studies, confirm unidirectional airflow patterns in an aseptic processing facility.

Integrated computerized systems for data collection improve data security and offer a solution for handling temporary data.

A skilled workforce is needed to deliver on technology’s promising medical advances.

Bioprocess understanding, the right equipment, and automation help, but multifunctional teamwork is the key to API production success.

The working acceptance limits for acceptance values (AV) are determined using the critical values at, for example, 95% coverage over the corresponding AV distributions. However, validity of such limits needs to be elaborated.

FDA’s commissioner addresses opioid abuse, drug costs, and manufacturing quality.

CDMOs can claim credit for the robust growth of emerging bio/pharma financings.
Monetary benefits will outweigh the hassle of batch record harmonization, says Siegfried Schmitt, principal consultant at PAREXEL.

Shipping biopharmaceuticals in single-use containers requires a thorough understanding of the distribution cycle and potential transportation risks.

The approach presented in this study uses process capability index results to establish sampling strategies for use with new product cleaning and to efficiently reduce the risk of insufficient cleaning.
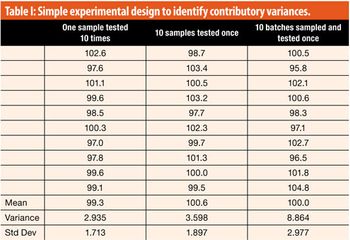
This article looks at a simple structured approach to assigning variance contributions and to assuring that the analytical procedure is fit for purpose.

Increased use of single-use systems has led to a need to redefine safe, stable and integral systems for shipping biopharmaceuticals around the world. This article provides qualification data under international ASTM D4169 norms.

Click the title above to open the Pharmaceutical Technology May 2018 issue in an interactive PDF format.