
The Easy Control Box from Mettler Toledo connects any lab sensor and pump to make real-time measurement and controlled dosing available to non-experts.

The Easy Control Box from Mettler Toledo connects any lab sensor and pump to make real-time measurement and controlled dosing available to non-experts.

The Ross PowerMix Planetary Dispersers with PLC Recipe Controls from Ross, Charles & Son deliver batch-to-batch consistency in the mixing of high-solids, high-viscosity applications.

The SMARTDAC+ GX series panel-mount type paperless recorder from Yokogawa Electric Corporation comes with new functions, including sampling intervals as short as one millisecond and the control and monitoring of up to 20 loops.

The Robotic Pulsed Light Sterilizer (RPLS1) from Steriline uses pulsed light technology for ready-to-use nest sterilization and can sterilize tubs of syringes, vials, and cartridges.

As cost pressures mount as a result of multiple biologics being developed for the same indication, manufacturers can harness process efficiencies to maintain the value of legacy products.
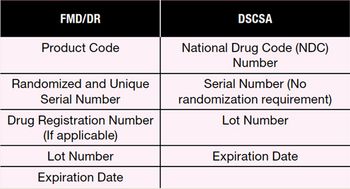
The author discusses upcoming serialization and transaction data collection regulations including the US Drug Supply Chain Security Act, the European Union Falsified Medicines Directive, and the EU Delegated Regulation.

Serialization and complementary authentication technologies are needed in order to meet DSCSA and FMD regulations.

The past several months have seen new product releases and updates made to already available laboratory equipment.

Industry experts discuss best practices for dissolution testing of poorly soluble, immediate-release, and controlled-release formulations and the different analytical approaches used.

Modern air jet milling can be used to investigate the feasibility of micronization as a solubilization approach in formulation development.
In this study, the authors describe a forensic microscopy approach to characterize particles that were visually observed during stressed stability testing of an ophthalmic solution formulation.

The impact of Brexit on the European drug approval regulatory framework presents challenges for EMA.

Follow guidelines for E&L studies of an orally inhaled and nasal drug product formulation in its delivery device.

Approval of breakthrough therapies requires expedited quality assessment.
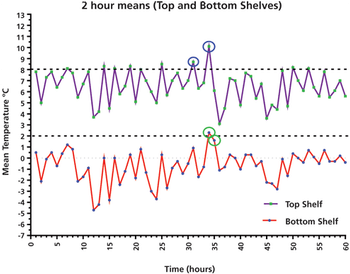
This column presents a data case study of a laboratory refrigerator and its qualification performance over five days, with important lessons for using average and individual results, as well as user requirements.

The authors summarize the current regulatory expectations regarding the number of PPQ batches required and provide potential approaches that can be used to determine and justify the number of PPQ batches.
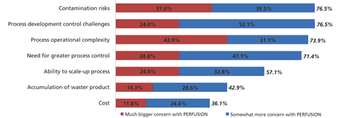
Although widespread adoption of continuous bioprocessing has been slow, some processes have been an exception.

Informing your clients of possible changes in equipment is imperative when upgrading a laboratory, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

Single-use systems demonstrate advantages over stainless-steel systems for biopharmaceutical manufacturing facilities.

Efficient synthesis of complex cannabinoids is possible while avoiding marijuana cultivation.

New reports indicate that drug prices are slowing compared to other healthcare costs.

Click the title above to open the Pharmaceutical Technology June 2017 issue in an interactive PDF format.