
CMOs expand capacity and capabilities in high-potency manufacturing to meet strong demand for cytotoxic and other potent drugs.

CMOs expand capacity and capabilities in high-potency manufacturing to meet strong demand for cytotoxic and other potent drugs.

Patent pressures, changing disease profiles, and higher costs force companies to fight for the top.

Agents report unusual chemistry, abnormal data analysis, and unconventional work practices.

This year's meeting of the Controlled Release Society unveiled a plethora of research insights.

Myth versus Reality: What does Q10 implementation really mean for my company?
Editors' Picks of Pharmaceutical Science & Technology Innovations

Surrounded by competition, Vietnam's 2020 vision focuses on building a biotech sector worthy of its Asian neighbors-as well as the growing global biopharmaceutical market

Legislative decisions to increase Medicare's formulary may lead to a fight over drug approvals.

USP's guideline for pending monographs can speed up publication of monograhs and time to market.
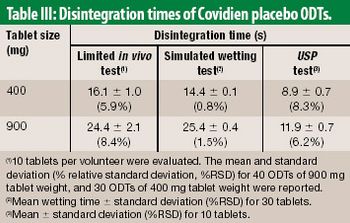
The authors propose an alternative to the USP disintegration test method. The method embraces physiological conditions of the oral cavity, as a screening tool for developing ODT products.

FDA is modernizing adverse-event reporting as part of a revolution in drug-safety assessment.
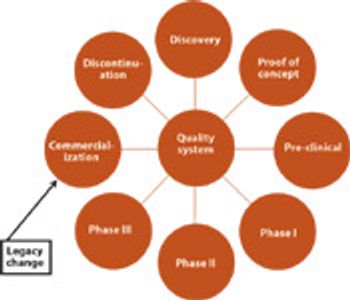
After five years in the making, the official pharmaceutical quality system is here. All three parties to ICH adopted a final version of Q10 and agreed to implement the guideline through their individual regulatory bodies.

CMC service providers are doing well, but clinical and preclinical CROs are doing even better.

Postponement of California's deadline gives the supply chain time to refine ePedigree solutions.

Despite its flaws, a recent release fills a need for books about pharmaceutical project management.

The authors consider several common techniques for verifying the accuracy of liquid-handling equipment and offer guidance for finding the appropriate technique for a given instrument.
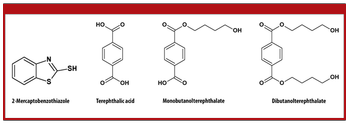
Extractables and leachables are a growing concern for pharmaceutical manufacturers and regulatory bodies.