
NIH leads new international consortium to develop a diagnostic tool for most known rare diseases by the year 2020.

NIH leads new international consortium to develop a diagnostic tool for most known rare diseases by the year 2020.

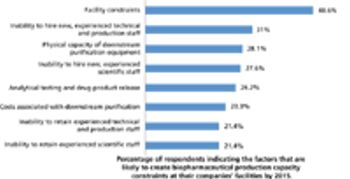
Rising imports and overseas production spur realignment of enforcement.

The pharmaceutical majors continue rationalizing manufacturing capacity in established markets as they forge their manufacturing networks in biologics and emerging markets.
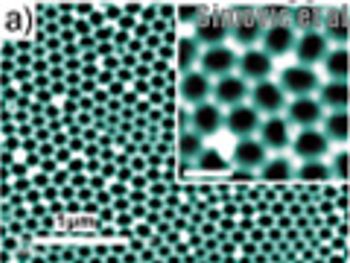
The authors present a method for controlling the release of therapeutics by applying a plasma polymer layer to the surface of porous materials.

Contract manufacturing organizations throughout Asia are increasing their capabilities to meet market demand and attract foreign investment and partnerships.

Are biosimilars the next big thing or just the next big bubble?

O-arylation and O-alkylation, a one-pot protein synthesis, a combined approach in continued and chemocatalysis, and green-chemistry applications are the target of some recent advances in API synthesis.
China rises to the top as a destination for international outsourcing.

A firm grasp of probability and ongoing re-evaluation are key.

A book about developing quality-control training manuals provides a wealth of information and a dearth of practical help.

New product reviews for August 2011 focusing on automation, process control, and information technology.
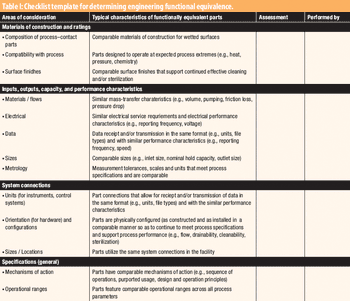
The second in a series of eight case studies from the Product Quality Research Institute focuses on functional equivalence for equipment.

Visiting a new site or going down memory lane may not get you where you want to go.

New studies reveal the promise and feasibility of transdermal vaccine delivery.

Many child-safe package designs help improve compliance and provide tamper evidence.

This study demonstrates the beneficial use of a spatial-filter velocimetry particle-size analyzer during granulation.

Terry Novak, president of Norwich Pharmaceuticals, on recent industry trends.
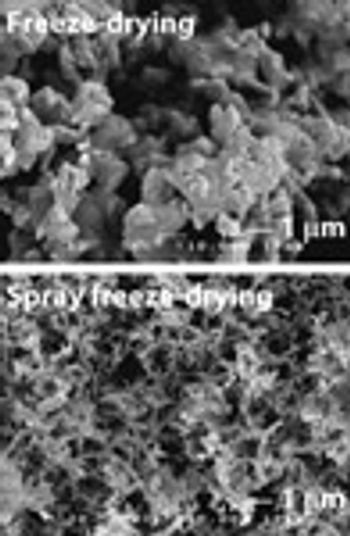
The authors discuss the preparation of lipophilic drug nanocrystals by controlled crystallization during freeze-drying.

Quality management requires more effort in a complex supply chain.

Thomas P. Layloff describes the advantages of using thin-layer chromatography methods for counterfeit detection. This article contains bonus online material.

Analytical detection techniques help combat counterfeit drugs.

A path to personalized medicines creates a new paradigm for development and manufacturing.

USP promotes horizontal standards for biologics' quality attributes.

Click the title above to open the Pharmaceutical Technology August 2011 issue in an interactive PDF format.