
Measuring Growth - Big Pharma's Manufacturing Investments

Measuring Growth - Big Pharma's Manufacturing Investments

A Q&A with Babu Padmanabhan, Managing Director and Chief Knowledge Officer of STEER Engineering, on recent industry trends.

Manufacturers willing to report bad news about the supply can help reverse the shortage trend.

After a series of government reforms that are appealing to both domestic and foreign players, the Japanese pharmaceutical market is making a comeback.

The author discusses strategies for preventing cargo theft.

Approaches to scaling up API syntheses center on ways to optimize process conditions and operability.

A look at elastomer changeout times shows how industry knowledge improves operations and cost.

Pharmaceutical Technology's annual manufacturing investment update shows slight gains in biopharmaceutical manufacturing and emerging markets and continued restructuring of supply networks.
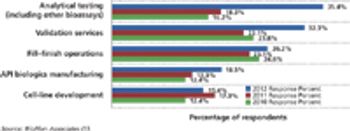
Budgets for biopharmaceutical activities are gaining in select functional areas except outsourcing.

New product reviews for August 2012.

Import controls and risk strategies aim to promote quality and spur new drug development.

The authors compare direct combustion with rinse and swab sampling methods.

The author discusses how to manage pending pharmacopeial changes.

No matter the upside or downside to the Affordable Care Act, there's work to be done.
Enhancing bioavailability can be achieved through hot-melt extrusion or spray drying. Patricia Van Arnum interviews Bend Research to find out more about when to use each technique.

Applying the recommendations of ICH Q10 to statistical analysis can help prevent product recalls.

Highly automated and sensitive quality-control equipment quickly identifies product faults.

IQ Consortium representatives explore industry approaches and practices for applying GMPs in early development.

New law provides FDA with the resources it needs to safeguard drug supply chain.

Meticulous system configuration can prevent machines from taking over.