
Contract research, development, and manufacturing organizations (CROs, CDMOs and CMOs) are embracing lean manufacturing, while Big Pharma is applying it, not so much for inventory management, but to improve supply chain visibility and control.

Contract research, development, and manufacturing organizations (CROs, CDMOs and CMOs) are embracing lean manufacturing, while Big Pharma is applying it, not so much for inventory management, but to improve supply chain visibility and control.

A study of root cause in stability samples suggests the need for tighter control of the sodium lauryl sulfate manufacturing processes.

Engineered containment performance testing is a more robust method for validating containment systems than worker-exposure measurement methods.

Paperless operations improve efficiency and increase assurance of product quality.

Choosing the correct shipping solutions, including packaging, transportation mode, and monitoring, helps mitigate the risks inherent in global logistics.
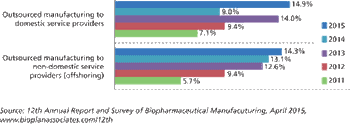
Biopharma companies are outsourcing more jobs to cut costs.

The trend towards personalized medicines in Europe requires a more integrated framework that regulates the approval of devices and diagnostics.

FDA and industry support global framework and collaborations to secure the supply chain.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general council, both of Regulatory Compliance Associates, discuss the requirements for a successful corrective action and preventive action (CAPA) system.

FDA notes progress in drug development, but cites scientific and funding roadblocks.

Potential for improved product quality and cost/time savings is reviving interest in perfusion technology.

Mitigating risk in the bio/pharmaceutical sector demands a holistic approach.

The Ross Inline Solids/Liquid Injection Manifold (SLIM) Mixer is designed to work with a range of powders that are challenging to mix into liquids.
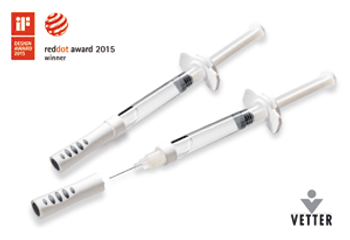
Vetter’s Vetter-Ject closure system for prefilled syringes has an integrated needle hub and shield and is designed for sensitive compounds, such as biologics.

Shimadzu’s LCMS-8060 triple quadrupole mass spectrometer is designed for LC/MS/MS quantitation of highly sensitive applications.

The YDP30 printer from Sartorius automatically performs thermal transfer or direct thermal printing, without the need to change settings, to meet requirements for fade-resistant printout in regulated areas, such as quality-control laboratories.

Click the title above to open the Pharmaceutical Technology August 2015 issue in an interactive PDF format.

China’s emergence as a significant commercial market is forcing manufacturers to re-evaluate their overall business model.