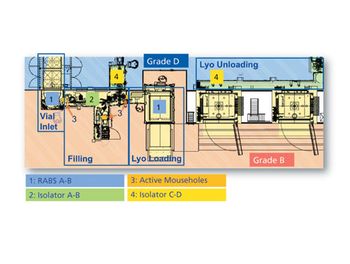
The design of Baxter BioPharma Solutions’ aseptic filling lines provides a case study in customizing containment systems for multi-product lines

The design of Baxter BioPharma Solutions’ aseptic filling lines provides a case study in customizing containment systems for multi-product lines

Three-dimensional printing allows unique benefits to be built in to solid-dosage forms.

Susan Schniepp, Distinguished Fellow at Regulatory Compliance Associates, discusses the regulatory requirements for improving manufacturing lines.
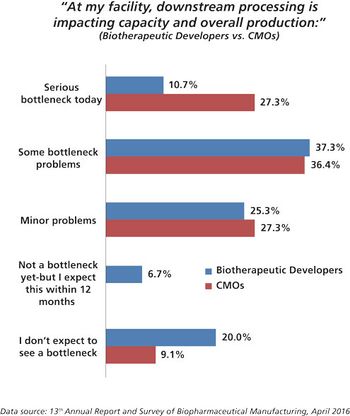
CMOs are working hard to improve performance by investigating new technologies for filtration and purification.

Regulators and manufacturers address economic and ethical issues for scarce medicines.

New technology introduced in 2016 aids innovation growth for pharmaceutical manufacturing.

A technology management process identifies and evaluates new technologies in biopharmaceutical manufacturing to aid business decisions.

A Q&A with FDA to gain insight on FDA's views of three-dimensional printing and its regulation in drug manufacturing.

PharmTech’s 2016 survey shows general satisfaction with existing solid-dose and parenteral manufacturing equipment, and slow adoption of continuous manufacturing processes.

Airlines, airports, freight forwarders, and other cold-chain partners are taking a crash course in pharma cGMPs.
Industry experts discuss key considerations in the development of orally disintegrating tablets.

Enzymatic catalysis offers pharma manufacturers a way to implement the Principles of Green Chemistry.

Recent revisions to the European Union’s good distribution practices, which were updated in 2013, reflect increased regulatory focus on the supply chain.
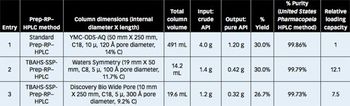
This paper describes a unique Prep-rP-HPLC technique that uses a C-18/C-8 derivatized silica coated with a hydrophobic quaternary ammonium salt or quaternary phosphonium salt that acts as an additional/surrogate stationary phase (AsP/ssP).

The rotary lump breaker unit from Ajax Equipment comprises a screw feeder with sifting screens and collecting conveyor for a gentle lump breaking action on pharmaceutical powders.

The Titan Syringe Pump from Syrris is a continuous flow chemical processing module suitable for lab-, pilot-, and production-scale applications.

The Ross SysCon Portable Pumping System is capable of handling laminar and turbulent flow applications across a 26-element low pressure drop static mixer.

The IsoBag from MilliporeSigma is used for the convenient transfer of contact and settle plates to production isolators.

Conventional limit-setting techniques are not health-based and can make risk assessment more difficult.

Click the title above to open the Pharmaceutical Technology August 2016 issue in an interactive PDF format.

Congressional partisanship creates noise, but no funding for Zika virus research.

The pharmaceutical industry now braces itself for the consequences and complexities that could follow in the wake of the United Kingdom’s decision to leave the European Union.