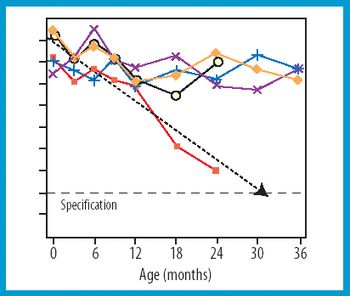
In a follow up to its 2003 partner article, the authors suggest three different levels of out-of-trend stability data and discuss issues surrounding the identification and investigation of each level.

In a follow up to its 2003 partner article, the authors suggest three different levels of out-of-trend stability data and discuss issues surrounding the identification and investigation of each level.

Formulation residue, observer viewing distance, light intensity, viewing angle, observer viewing position, and observer-to-observer variability affect the ability to confirm the cleanliness of manufacturing equipment.
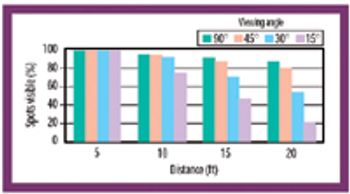
Formulation residue, observer viewing distance, light intensity, viewing angle, observer viewing position, and observer-to-observer variability affect the ability to confirm the cleanliness of manufacturing equipment.
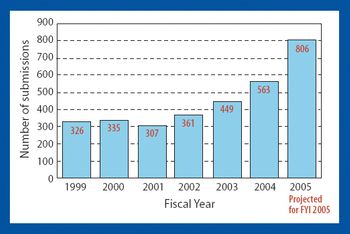
The US Food and Drug Administration (Rockville, MD, www.fda.gov) released a new draft guidance that may speed generic approvals. The guidance, ANDAs: Impurities in Drug Products, describes the degradation-product information that generic drug manufacturers should include in their abbreviated new drug applications (ANDAs). This clarification, FDA officials say, will help companies submit the correct information, thus increasing the likelihood that their generic drugs will be approved, and approved more quickly.
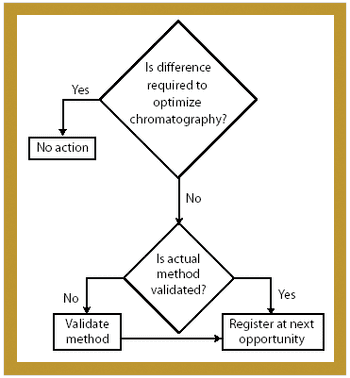
Manufacturers can take steps to establish a regulatory compliance assessment program in their pharmaceutical manufacturing facilities.

For years, pharmaceutical manufacturers have complained that federal regulations impede process improvements. Now, FDA is trying to do something about it.

Ensuring access to quality medicines while ramping up production requires attention to supply chains, bioequivalence testing, and patent and regulatory issues.
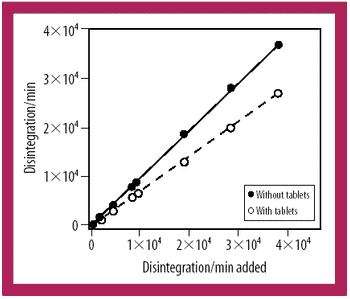
A radiotracer technique is a simple, fast, and sensitive technique for analyzing the integrity of clinical supply packages to water.

Although patient compliance problems have been receiving attention for at least a decade, many medications are still dispensed in bottles that contain a supply intended to last days or weeks and require considerable effort on the part of the patient or caregiver to keep track of the dosing schedule. As a result, when it comes to consistently taking the right dose at the right time for the duration of a prescription, many consumers don't do a very good job.

Growth is critical to a strong company, and not only because it generates economies of scale.

Confronted by a challenge as vast as Hurricane Katrina, we reach for military organizations for aid and military language for description.
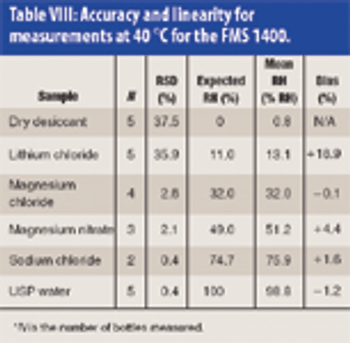
Frequency modulation spectroscopy is a nondestructive technology for determining the water activity of pharmaceutical samples. This article discusses the various pharmaceutical applications of frequency modulation spectroscopy, offers comparisons with various traditional water activity measurement techniques, and presents an assessment of various instrument performance elements.

It was, our GMP Agent-in-Place recalls, a typical, small, clinical-type facility... managed in the typical, informal way.

The translator strives for quality, for a flawless re-setting of ideas from language to language. It is precise, painstaking work, making sure that all the concepts are there, that the grammar and syntax are correct, that the register (the formality, the emotional and social tone) is accurate, and that the text reads smoothly.

Two scenarios demonstrate the need to use the percent of parent drug loss rather than the percent of degradation products formed when reconciling mass balance calculations.

As overseas sourcing arrangements become more and more common, it may be time to look again at the advantages of having suppliers closer to home.

This review article discusses orally disintegrating tablets and their manufacturing technologies, development issues, and future trends.
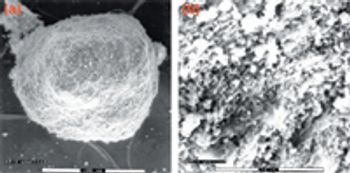
Although agitation improves drying efficiency and ensures uniformity of the final dry material, it can also affect the physical properties of the product as it dries. This study evaluates the effect of scale up and equipment selection on an active ingredient undergoing granulation during the drying process.