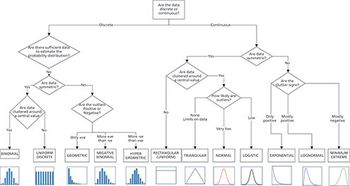
The new Fourier-Transform Infrared (FT-IR) spectrometer from Protagen Protein Services is used for advanced structural characterization of protein-based biologics and monitoring of vibrational modes of the amide bonds within a protein while in its native state and formulation matrix.