
More complex and challenging compounds require a more tailored approach to formulation strategies.

More complex and challenging compounds require a more tailored approach to formulation strategies.

This article describes a validated method, using liquid chromatography/mass spectrometry, for detecting the carcinogenic impurity NDMA (N-nitrosodimethylamine) in ranitidine that meets and exceeds FDA’s limit of detection and limit of quantitation requirements.
Taste-masking can be of significant importance in ensuring success of a drug product, particularly those targeted to pediatric or geriatric populations.

Excipients must be carefully chosen to ensure optimum protection for vaccines and live biotherapeutic products.

Research is striving to make pharmaceutical processes more scalable by making them simpler and easier to replicate and control. Advances include 3D printing, as well as miniaturized and continuous processes, all of which are being aided by improved automation and analytics.

The manufacture of gene therapy vectors is shifting to more modern technologies.

Industry experts discuss challenges and best practices for scaling up manufacturing under a short deadline.

The case for migrating from a paper-based quality management system to a digital platform is presented.

A comprehensive rewrite of Annex 1 has been proposed and aims to organize and structure requirements in 10 specific sections.

Facility and equipment design are important, but the team and its experience matter most.
End-to-end traceability can provide more value than just securing drug product safety.

Cellular and gene therapy fields are currently on track for, if not already experiencing, a serious capacity crunch.

Drug shortages and supply chain challenges bolster FDA efforts to promote modern manufacturing.

Regulatory bodies are adapting to new ways of working to cope with the impact of COVID-19.

Misleading messages contribute to eroding trust in public health agencies.

European pharma companies may have a vested interest in the outcome of the US elections.

The amount of detail included in SOPs may help a company stay compliant, says Susan J. Schniepp, Distinguished Fellow at Regulatory Compliance Associates, LLC.

Cytiva announced that it is launching the Xcellerex Automated Perfusion System (APS), a device aimed at simplifying processes and reducing risks when intensifying upstream operations.

The ROSS VMC-1000 VersaMix is a 1000-gallon triple-shaft mixer available on a pivot-design single-post hydraulic lift.
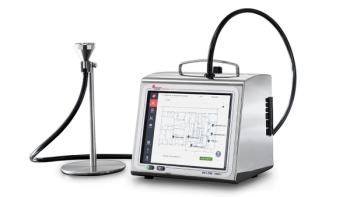
Beckman Coulter Life Sciences announced that its MET ONE 3400+ portable air particle counter is now available for cleanroom compliance.

The Spectrum Compact CE System from Promega is a benchtop capillary electrophoresis (CE) instrument that allows users to perform sequencing and fragment analysis at the bench in laboratories of all sizes.