
Manufacturers face regulatory overhaul, while brand-generic debates escalate over biosimilars and labeling changes.

Manufacturers face regulatory overhaul, while brand-generic debates escalate over biosimilars and labeling changes.

Ebola Virus Disease (EVD) is a severe, often fatal disease that is transmitted human-to-human through bodily fluids.
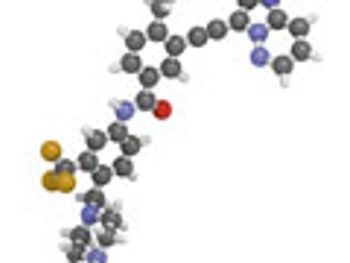
New practical approaches to the synthesis of complex heterocycles are reviewed.

EMD Millipore's Steritest Symbio Pumps are designed to create more reliable sterility testing in laminar flow hoods, isolators, and clean rooms.

Resolving the problem will require more communication between the industry and regulatory bodies.
The author reviews the challenges in delivering macromolecule biologics.

Connect 2 Cleanrooms' mobile, adjustable cleanroom filtration system, HEPA-lite, provides localized filtered air.

Using flow sensors, operators can monitor compressed-air use to help identify problems early and provides data for improving energy efficiency.
The parenteral manufacturing industry is taking action to address particulate contamination issues.

The authors explore and define common industry approaches and practices when applying GMPs in early development.

The authors take a look at some of the recent developments in the German pharmaceutical market.

A G-CON, GEA, and Pfizer collaboration developed a PCMM (portable, continuous, miniature, and modular) system to produce oral solid-dosage drugs.

Defining critical parameters and processing large quantities of data can be a challenge.

Measuring syringe plunger force using a texture analyzer instrument.
Enterprise quality management systems can help shift the quality emphasis from corrective to preventive actions.
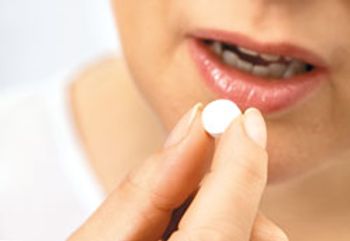
This review highlights relevant physicochemical drug properties and formulation design considerations critical to quality and performance of the sublingual tablets.

Telstar's ionHP biodecontamination system is designed to use ionized hydrogen peroxide in aseptic processes.

Nitritex introduces BioClean BarrierPlus isolator and Restricted Access Barrier Systems sleeves and gauntlets designed for highly-controlled environments.

Click the title above to open the Pharmaceutical Technology November 2014 issue in an interactive PDF format.

European CDMOs want into the US market, but entry options are limited.