
Problems in an induction-sealing process, such as untorqued or crooked caps, can be identified and corrected in real time using dynamic thermal imaging.

Problems in an induction-sealing process, such as untorqued or crooked caps, can be identified and corrected in real time using dynamic thermal imaging.
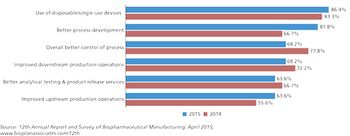
Better process development is creating industry benchmarks for bioprocessing.

New program emphasizes quality, risk, and global collaboration.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to ensure archive records can be retrieved.
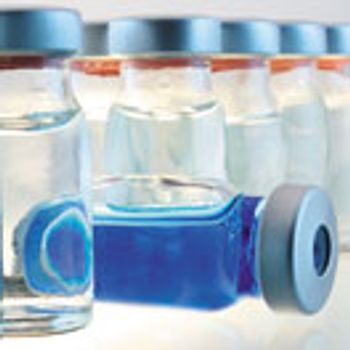
Delamination of glass packaging is a source of particulates in parenteral drugs, but identifying the root cause allowed the design of an improved manufacturing process for glass vials.

Virtual pilot programs examine scenarios that may occur while implementing serialization requirements for the US Drug Supply Chain Security Act.

Efficient syntheses are possible using multi-component and cross-dehydrogenative, heteroaromatic C–H silylation reactions

The scheme aims to ensure that EMA and licensing authorities of EU member states will use the same IT system, based on a single data standard.

Industry experts share insights on the advances in tablet coating technologies and the potential of continuous coating in solid-dosage manufacturing.

The determination of topical bioequivalence requires a multi-faceted approach, tailored specifically to the generic-drug formulation.
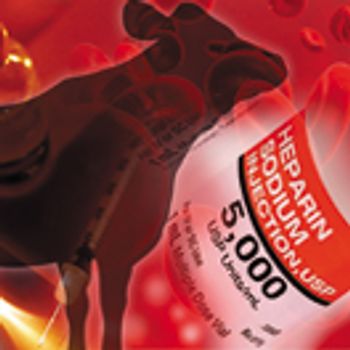
The global supply chain for bovine and porcine heparin and regulatory considerations are examined.

Sartorius Stedim Biotech’s Flexsafe 3D Pre-designed Solutions meet ASTM D4169 Standard Practice requirements and are designed for storage and shipping of biopharmaceutical fluids.

IMA Active’s new tablet press machine, Prexima, is designed to deliver higher productivity and efficiency.

Ross’ Batch High Shear Mixer features a four-blade rotor running within a close-tolerance stator at variable tip speeds up to 3000-4000 ft/min.
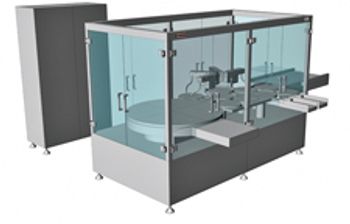
Pharmaceutical inspection machines from Bosch Packaging Technology include the KHS 1, which uses laser headspace analysis (HSA), and the AIM 3, which uses high-voltage leak detection (HVLD), for container closure integrity testing.

The CPhI Pharma Awards celebrate innovations in the pharma industry.
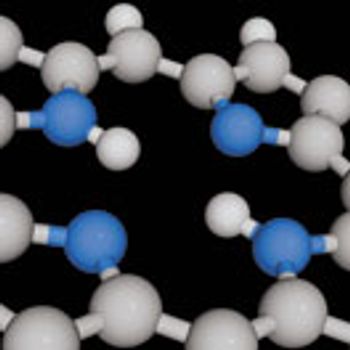
Efficient syntheses are possible using multi-component and cross-dehydrogenative, heteroaromatic C-H silylation reactions.

Although shortages, quality, and regulatory challenges remain, improved technologies and new investments suggest that the worst may be over.

Miriam Beyer, European marketing manager, West Pharmaceutical Services, describes causes of recent parenteral drug shortages.

Click the title above to open the Pharmaceutical Technology November 2015 issue in an interactive PDF format.