
The European Commission’s effort to relax supplementary protection certificates to help generic-drug makers and biosimilars producers has sparked strong opposition from the research-based pharmaceutical sector.

The European Commission’s effort to relax supplementary protection certificates to help generic-drug makers and biosimilars producers has sparked strong opposition from the research-based pharmaceutical sector.

Softgel capsules are a popular dosage form among patients but they also provide a number of manufacturing benefits over liquid-filled hardgel capsules.

Modified-release lipid-based formulations in softgel capsules can address physiochemical and pharmacokinetic challenges posed by drug compounds.

SOPs need to reflect a company’s specific manufacturing or other operations, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

New gene therapies and combination products require innovative regulatory approaches.

In a productive year, 2017 was filled with acquisitions, facility expansions, and new biopharma technology.

In a productive year, 2017 was filled with acquisitions, facility expansions, and new biopharma technology.

Optimizing water and HVAC systems can reduce resource use in all pharma facilities, and, for biopharma manufacturing, the supply chain of consumables should be evaluated.

Rinse sample analysis or visual inspection are risk-based approaches that can be correlated to surface cleanliness to replace surface sampling in a biopharmaceutical equipment cleaning process.

Attracting and retaining qualified bio/pharma experts demands a mix of recognition, rewards, and opportunities.

Is pharma up to the task of developing knowledgeable, motivated employees?
For many processes involving hazardous chemistry, running in flow mode has safety and economic advantages.

Amid business and regulatory uncertainty, bio/pharma experts reveal opinions on salary, recognition, and training.

A QbD approach can address manufacturing complexities in transdermal patch manufacturing.

Medherant has developed an improved instrument for testing drug release from transdermal patches.
Cross-functional reliability rooms identify risk and planning metrics, provide insights for production forecasts, and predict trends and areas for improvement.

A technology roadmap aims to drive and consolidate improvements in a process that has remained unchanged for more than 70 years.
In Part I of this article series, the authors discussed the regression control chart method for identifying out-of-trend data in pharmaceutical stability studies. In Part II, the by-time-point method and the multivariate control chart method are investigated, and improved approaches are suggested. The method is illustrated using real data sets.

The Hiden ExQ quantitative gas analyzer is a mass spectrometer system providing continuous on-line analysis of dynamic gas streams at pressures from sub-atmospheric up to 30 bar.
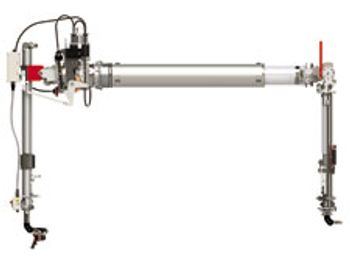
The VERSA Telemanipulator from Central Research Laboratories (CRL), a Destaco company, is suited for remote-handling applications that involve specific customization requirements.

The Ross, Charles & Son Ross X-Series is a clean-in-place (CIP) ready ultra-high shear mixer for inline emulsification, particle size reduction, and homogenization.

The InMotion Karl Fischer (KF) Oven Autosampler from Mettler Toledo is suited for KF titration and comes in two models, InMotion Flex and InMotion Pro, and allows up to 26 samples to be analyzed on a 25-centimeter platform.

Click the title above to open the Pharmaceutical Technology December 2017 issue in an interactive PDF format.