
Poor API quality may often lead to delays in production and a shortage of supply.

Poor API quality may often lead to delays in production and a shortage of supply.

Scientific, economic, and practical factors should be considered when choosing between the frozen state and lyophilization.
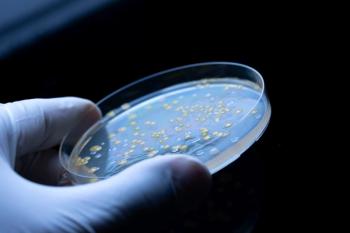
This article explores the impact of test volume, microbial distribution, and dilution errors on bioburden testing variability. It presents statistical approaches to estimate percentage error and discusses strategies to optimize microbial enumeration techniques in biopharmaceutical quality control.

Incorporating sustainable practices into process designs as early as possible ensures optimal performance.

The shift toward personalized medicines poses new challenges in cleanroom protocols.

The pandemic made it daunting for companies to retain talent and then find it anew, but just as that cloud is lifting and workforce diversity is being embraced, AI beckons as a new challenge.

The European Union is discussing ways to reduce Europe’s over-reliance on imports of APIs.

While global harmonization exists, there are still differences between the US and European GMP requirements that manufacturers should know, says Siegfried Schmitt, PhD, vice president, Technical at Parexel.

The pharma industry is focused on strengthening its foundation, embracing innovation, and future-proofing its path forward.