
The project will foster the translation of nanomedicine applications for the treatment of cardiovascular diseases.

The project will foster the translation of nanomedicine applications for the treatment of cardiovascular diseases.

The agency cited the company’s India facility for batch failures and data integrity problems.

The new guide offers guidance on how to ensure data and records are complete, consistent, secure, accurate, and available throughout their lifecycle.

The agency issued its recommendation for the influenza virus strains European vaccine manufacturers should include for 2017.

The company announced the expansion of its global shipping program, which now allows companies to ship dangerous goods between 36 countries.

Sharp acquired the pharmaceutical packaging facility in Bethlehem, PA.

Uhlmann Packaging Systems joined the OPC Foundation’s Open Serialization Communication Standard (OPEN-SCS) Working Group.

GE Healthcare continues to ramp up its offerings in the bioprocessing space with the purchase of Asymptote and a continued partnership with Zenith Technologies.

In a new study, researchers from Boston Children’s Hospital study responses to pneumococcal vaccine in infant monkeys.

The conference has partnered with Mercy Ships, a non-profit organization using hospital ships to deliver health care to developing nations.

Pharmaceutical Technology spoke with CPhI North America presenters Ben Locwin, PhD, MBA, MBB, president at Healthcare Science Advisors, and Tom Fox, principal at Advanced Compliance Solutions, to discuss drug pricing, compliance challenges, and corporate social responsibility in the bio/pharmaceutical industry.

A drop in US drug approvals was noted but this trend was not yet seen in Europe.

The recall was issued because of a defective delivery system; the units affected had possible package leakage.

MilliporeSigma’s new high-area cartridge filters and single-use capsules are suitable for feed streams with high levels of particulates.

The agency released guidance on single assessments of PSURs to improve safety and benefit-risk assessment of medicines.

The companies will work to co-develop FIN-524, a live biotherapeutic product composed of cultured bacteria strains.

Janssen will have access to PeptiDream’s proprietary Peptide Discovery Platform System technology, which will be used to identify peptides against multiple metabolic and cardiovascular targets.

The role of patient advocates in shaping regulations and policy has put attention on financial and operational links between drug companies and independent health organizations.

FDA is in the center of the debate over developing and pricing new cancer therapies.

A new study in NEJM compares the regulatory review processes of FDA and EMA.

Scott Gottlieb answers Senators' questions at his confirmation hearing before the Senate Health, Education, Labor and Pensions Committee.

Deutetrabenazine is the first deuterated product approved by FDA, approval represents the first new treatment option for chorea associated with Huntington’s disease in nearly a decade.

EMA has developed a framework and action plan to foster relationships with the academic community.

Sartorius acquired the Swedish company that specializes in data analytics software for biopharmaceutical development and manufacturing.

Carglumic acid is used in the management of rare, life-threatening inborn metabolic disorders affecting the urea cycle.

The company announced that Meridian Medical Technologies is extending a recall of EpiPen and EpiPen Junior to the United States.

The company has invested GBP9 million (approximately $11.6 million) to fund a new multiple-phase pharmaceutical manufacturing, packaging, and distribution facility in Wales, United Kingdom.

GW Pharmaceuticals plans to submit a regulatory filing to FDA and EMA following two positive Phase III trials of Epidiolex in patients with Lennox-Gastaut Syndrome.
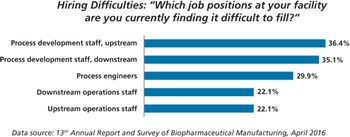
New study shows China biopharma companies face staffing shortages.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the value of internal audits and how the information gained can be applied.