
BPS-804, which is being developed for the treatment of osteogenesis imperfecta (brittle bone disease), has been granted orphan drug designation by both FDA and the European Commission.

BPS-804, which is being developed for the treatment of osteogenesis imperfecta (brittle bone disease), has been granted orphan drug designation by both FDA and the European Commission.

Recipharm’s addition of Kemwell’s Bengaluru, India facility expands its solid- and liquid-dosage capabilities.

Sartorius and EMBL have entered into a corporate partnership program to foster advanced training.

The ICH Q11 Q&A discusses the development and manufacture of drug substances and the selection and justification of starting materials.

SGS expands its elemental impurity testing services at its laboratory in Villeneuve-la-Garenne, France.

PhRMA submits comments to the The Office of the United States Trade Representative encouraging protection of US innovation in foreign markets.

FDA sent a warning letter to Sato Pharmaceutical Co., Ltd. after inspectors found deviations in the facility’s aseptic processes.

FDA approved Valeant’s brodalumab with a boxed warning for suicidal ideation.

The Patent Trial and Appeal Board ruled in favor of the Broad Institute, allowing the institution to keep patents for its CRISPR-Cas9 gene-editing technology.

The new commercial site, set to be located in Cambridge, Boston, MA, will serve clients on both the East and West Coast. It will also be the base for reaching new customers in the area.

The acquisition complements Catalent’s global OTC and prescription pharmaceutical softgel capabilities and capacity.

South East Asian pharma manufacturers seeking region growth expected to attend CPhi South East Asia.

Process conditions can corrode stainless-steel surfaces, necessitating corrective and preventive maintenance.

The directorate says monographs are flexible and changeable and their compliance does not on its own determine biosimilarity in biosimilars.

The agency sent a warning letter to Resonance Laboratories Pvt. Ltd. after an inspection found possible contamination problems.

The company has voluntarily recalled all lots of of human chorionic gonadotropin because of a lack of sterility assurance.

Baricitinib (Olumiant) is the first JAK inhibitor licensed to treat rheumatoid arthritis in Europe.

Aurobindo has added four cell-culture derived biosimilars to its product line.

The Generic Pharmaceutical Association announces a rebranding campaign to expand access to medicines.
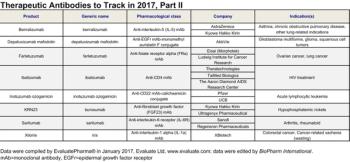
Evaluate and BioPharm International highlight the antibody-based therapeutics that may have 2017 launch dates in the United States.

Quotient Clinical’s addition of CDMO QS Pharma increases the company’s footprint in the US an adds high potency molecule capability.

The company announced results from a Phase II trial with its THC:CBD drug for the treatment of glioblastoma multiforme.

The companies entered into a license agreement for Immunomedic’s antibody-drug conjugate sacituzumab govitecan.

EvaluatePharma and BioPharm International highlight the antibody-based therapeutics that may gain United States Regulatory approval in 2017.

EMA announces that the European Union’s PAS Register has received its 1000th upload.

A total of 166 biotech executives penned an open letter expressing concern over President Donald Trump’s executive order on immigration.

Pharmaceutical Technology spoke with Tommy Fanning, head of biopharmaceuticals for IDA Ireland, to get a perspective on how Brexit may affect the pharmaceutical industry.

The company is voluntarily recalling product due to particulate matter.

Dr. Reddy’s has expanded its commercial operations in Europe with the introduction of its range of generic drugs in France.

Modular Automated Sampling Technology (MAST) allows direct aseptic transfer of bioreactor samples to analytical devices, providing rapid and reliable data in bioprocessing.