
Intellectual property lawyers estimate biosimilar litigation will swell as early as 2018.

Intellectual property lawyers estimate biosimilar litigation will swell as early as 2018.
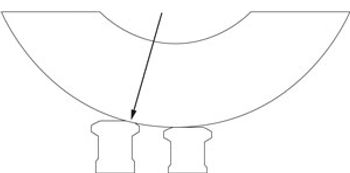
Adjusting the tablet press and its systems can prevent manufacturing and product quality problems.

Pharma can boast of big-picture successes, but needs to work on operational issues.

The restructuring of the International Conference on Harmonization, which is expected to begin in late 2015, could have a significant impact on the way pharmaceutical regulations are harmonized worldwide.

The revised "21st Century Cures” proposal includes provisions for research for continuous manufacturing, biomarker development, and NIH funding.

Perrigo rejected Mylan’s second proposal for an acquisition valued at more than $34 billion, saying that the offer was too low to consider.

Takeda Pharmaceutical agrees to pay $2.4 billion to settle lawsuits from patients and family members who said the diabetes drug Actos caused bladder cancer.

Pharmaceutical companies should take into consideration intellectual property protection when outsourcing the packaging of their products.

In a seething letter, Mylan’s executive chairman announced the unanimous rejection of a $40-billion unsolicited acquisition offer from Teva.

After rejecting Teva’s unsolicited $40 billion purchase offer, Mylan has increased its own offer to purchase Perrigo from $29 billion to over $32 billion in cash in stocks.

Mylan announces a recall of eight lots of injectable products due to visible foreign particulate matter.

FDA releases long-awaited guidance documents regarding the assessment of biosimilarity.

CDMO Vetter produces identifiable labeling for a top-ten pharmaceutical company.

IDT Biologika receives 2015 Facility of the Year Award or facility integration from ISPE.

Innate Pharma announces a co-development and commercialization agreement with AstraZeneca to accelerate the development of Innate’s anti-NKG2A antibody.

CPhI Russia opens in Moscow as the government seeks to boost domestic manufacturing production.

With the Indian pharmaceutical industry on the rise, manufacturing businesses are working together with European and American partners to harness their longstanding experience and reputation in cleanroom manufacturing for a broader pharmaceutical manufacturing marketplace.

Stability testing and UPLC capabilities highlight expansion at Mumbai, India facility of SGS Life Science Services.

Catalent’s Zydis technology will be used to develop thermo-stable and cold-chain independent vaccines.

Sonneborn announces that it named Gregg Kam as its new chief financial officer.

The agency has recommended granting marketing authorization for Opdivo.

Dicerna Pharmaceuticals announces that FDA granted its primary hyperoxaluria type 1 (PH1) treatment Orphan Drug designation.

The company voluntarily recalls Preservative-Free Bupivacaine HCl Injection, USP due to potential iron oxide particulates.
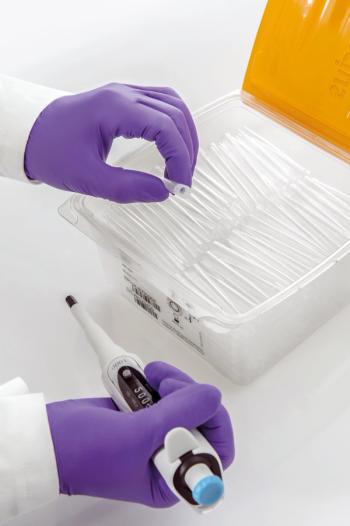
FlexiBulk tip packs save space and effort in the laboratory

MaxCyte announces a strategic partnership with Johns Hopkins University to develop CAR T-Cell therapies for cancer.

Originator product manufacturers will have to update and improve their processing platforms to stay competitive with the biosimilars coming to market.

Prequalified manufacturing suites could benefit from a new business model, say some industry executives.

INTERPHEX 2015 is under way and Pharmaceutical Technology and BioPharm International are in the middle of the action!

The past six months has seen some major changes to the sterile manufacturing landscape in Europe. There have been a number of exits and acquisitions that have no doubt grabbed headlines, but has anything really changed?

With the inauguration of the Technology Center, the engineers, scientists, pharmacists, and IT developers for the first time closed the loop control circuit of a modular system along the entire process including production, sensor technology and controlling.