
Mylan Pharmaceuticals launches Hertraz, the world's first trastuzumab biosimilar in India.

Mylan Pharmaceuticals launches Hertraz, the world's first trastuzumab biosimilar in India.

Novartis is named among the 2014 Corporate Knights Global 100.

Sartorius appoints Michael Melingo to head marketing, sales, and services of the Lab Products & Services Division.

Shimadzu's new Solution Center features additional research space.

In first official visit to India, FDA commissioner to discuss ongoing collaboration on drug programs.

BioOutsource will open a new Biosimilar Center of Excellence in Scotland.

Partners MPI Research, inviCRO, and 3D Imaging announce name of new facility for molecular imaging, autoradiography, and animal modeling.

NIH, 10 biopharmaceutical companies, and several nonprofit organizations form a partnership to develop new treatments earlier for Alzheimer's, type 2 diabetes, and autoimmune disorders.

Thermo Fisher Scientific outlines new business segments with acquisition of Life Technologies.

Neolpharma opens its new Development and Innovation Center (CEDIPROF) for pharmaceutical products in Puerto Rico.

Edison and DSP partner to develop drugs targeting cellular energy metabolism.

Last year, the Turkish Government unveiled plans to position the country in the top 10 most-preferred locations for R&D investment in the pharmaceutical sector by 2023. This article provides an overview of the incentives introduced under the Technopolis Law and the R&D Act.

PDA works with FDA to create pharmaceutical quality metrics.

ISPE and PDA take on the challenge of recommending quality metrics.
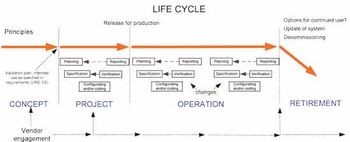
Non-compliance issues show that users find dealing with computer systems challenging.

PharmaCell enters an agreement purchase TiGenix therapy production facility.

BASF receives EXCiPACT Certification for the group of polyvinylpyrrolidone polymers, produced in Germany.

Mayne Pharma appoints Stefan Cross as president of its US division.

Solvias appoints Karen Huebscher as its new CEO.

I Holland announces service that predicts solutions for tablet sticking problems.

Jeff Yuen had been appointed executive vice-president, global quality for combined companies.

Regis Technologies passes a recent FDA audit with no Form 483 observations.

Optima pharma GmbH appoints Stephan Reuter as its new CEO.

Eurofins MWG Operon expands its exome sequencing services with the Ion Proton Next Generation Sequencer.

Avantor Performance Materials adds VWR International locations to its certified excipient distributor program.

AstraZeneca and Bristol-Myers Squibb Diabetes Alliance provides a $5 million grant to the American Diabetes Association.

International Society for Pharmaceutical Engineering will continue strategic plan initiated by Nancy Berg; search begins for replacement.

Teva reports FDA approval for three-times-a-week Copaxone 40 mg/mL.

Covance appoints Barry J. Goldstein as its new vice-president of Clinical Development Services and Global Therapeutic Area Head.

SGS to offer program-based stability testing services for biopharmaceuticals from Wokingham, UK facility.