
Prior to price escalation of pharmaceutical products in Brazil, the country's regulatory authority released a study on price-cap control and its benefits in the past years.

Prior to price escalation of pharmaceutical products in Brazil, the country's regulatory authority released a study on price-cap control and its benefits in the past years.

Drug companies team up with INTERPOL to keep counterfeit medicines off the Internet and out of the hands of patients.

FDA faces budget crunch; Supreme Court hears key cases
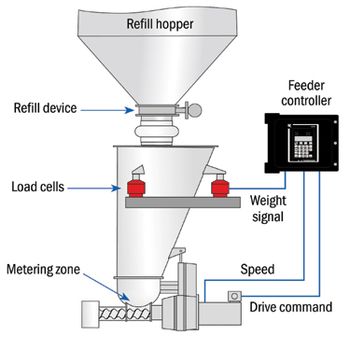
Loss-in-weight feeders provide high accuracy for batch or continuous processes.

Wanted: Article contributions on drug and process development topics.

Genentech announced that FDA has approved Actemra (tocilizumab) for the treatment of polyarticular juvenile idiopathic arthritis (PJIA) in children aged two years and older.

Pharmaco-Kinesis Corporation (PKC) announced that it is developing the first nanodrug combination of Merck's temozolomide and Celgene's thalidomide (in a 50:50 ratio) for the treatment of gliomas and other cancers. PKC will collaborate with the University of California, San Diego's department of nano-engineering at the Moores cancer centre to develop the nanodroplet formulation.

Bayer announced that it has signed a merger agreement with US-based birth-control specialist Conceptus.

Marketing agreement involves particle-sizing and milling equipment.

New company will focus on inspection, monitoring, and control systems for automated pharmaceutical manufacturing processes.

A personalized drug research and development platform will validate biomarkers for individualized disease treatment for Chinese patients.

Pharmaceutical Technology has joined with Dun & Bradstreet Credibility Corp. to launch the Pharmaceutical Technology and BioPharm International Marketplace.

Catalent Pharma Solutions, a provider of drug and biologic development services, delivery technologies and supply solutions, has officially opened a new biomanufacturing center of excellence in Madison. The facility, which was constructed in response to customer demand, is expected to quadruple Catalent's current biologics manufacturing capacity and extensively utilise single-use technology for greater flexibility and efficiency. It will allow the company to extend its offerings in the biologics sector while enhancing the efficiency and output of its proprietary GPEx cell line engineering technology as well as other mammalian cell lines.

FDA will use a new anticounterfeiting tool to detect fake medicines.

Lundbeck has launched Selincro in Norway, Finland, Poland and the Baltic countries of Latvia, Lithuania and Estonia for the treatment of alcohol dependence in patients with high-risk drinking levels. According to Lundbeck, the launch marks the first introduction of a new treatment for alcohol dependence in Europe for more than a decade.

BioOutsource, a contract testing services provider to the biopharmaceutical industry, has opened a new facility in Cambridge, Massachusetts. The facility marks the company's first North American offices, which were opened in response to increasing demand for the company's bioanalytical and biosafety services, particularly in the field of biologic and biosimilar characterisation.

FDA issues draft guidance to minimize medication errors.

Third compounding pharmacy recalls products due to FDA inspection.

EMA has upgraded its EudraGMP database to include information on GDP in addition to GMP. The new EudraGMDP database is a key deliverable of the Falsified Medicines Directive (FMD) that came into effect in January 2013. The aim is to increase supply chain security in the EU by making supervision of manufacturing and distribution of medicines more robust to ensure supplier compliance.

AstraZeneca and BIND Therapeutics have formed a strategic collaboration to develop and commercialise BIND's Accurin, a targeted and programmable cancer nanomedicine, based on a molecularly targeted kinase inhibitor developed and owned by AstraZeneca.

Metrics Inc. offers Cleantaste technology, which enables polymer coating of individual drug crystals to produce particles sized 25 um to 125 um.

Allergan has addressed FDA?s concerns with canister filling.

Unique technology expands Entegris? fluid-sensing and control offering.

Cipla has launched the first biosimilar of etanercept in India for the treatment of rheumatoid disorders. The product will be marketed under the brand name Etacept. The launch of Etacept marks Cipla's entry into the biologics market, offering a low-cost alternative to Pfizer and Amgen's rheumatic disorder blockbuster Enbrel in India.

Eli Lilly has announced positive top-line results for two of its Phase III trials, AWARD-2 and AWARD-4, which evaluated dulaglutide as a once-weekly treatment in patients with type 2 diabetes. Both trials met the primary efficacy endpoints, demonstrating non-inferiority of dulaglutide compared to insulin glargine, measured by a reduction of hemoglobin A1c (HbA1c) levels at the 1.5 mg dose level.

Oval Medical Technologies, an autoinjector company based in Cambridge, UK, reported that a variety of highly viscous solutions have been successfully delivered through a 25-gauge thin-wall needle, in less than 7 seconds, using its innovative autoinjector. The technology provides solutions to problems in the industry for drug containment and the end user.

FDA has released Guidance for Industry: Non-Penicillin Beta-Lactam Drugs: A CGMP Framework for Preventing Cross-Contamination.

The FDA approved updated labeling for Purdue Pharma L.P.?s reformulated OxyContin tablets, but also determined that it will not approve generic versions of the original OxyContin.

New manufacturing route improves availability of ingredient for malaria treatment.

Speakers discuss modularization, single-use systems, and process validation in a series of podcasts available on the Pharmaceutical Technology website.