
The facility in Hangzhou will package Merck medicines for China and the Asia-Pacific region.

The facility in Hangzhou will package Merck medicines for China and the Asia-Pacific region.

Amgen Inc. has agreed to pay the United States $24.9 million to settle allegations that it violated the False Claims Act.

$13.6 billion deal strengthens Thermo?s leading role.

FDA inspections of compounding pharmacies manufacturing sterile-drug products lead to voluntary recalls.

The parties partner for evidence-based formulations for emerging markets

New facility for Bio-Manguinhos will use plant-based protein expression technology.

FDA's Fiscal Year 2014 budget request includes more than $10 million above the 2012 budget for inspections of products manufactured in China.

The alliance will develop treatments based on Isis' antisense technology.

FDA inspections of compounding pharmacies manufacturing sterile products reveal non-sterile conditions.

Prefilled-syringe line features automation and novel disinfection techniques.

The company expands to add process-development and clinical-manufacturing capabilities at its large-scale bulk biologics facility.

The process-driven system reduces total cost of ownership.

Company is notified of GMP violations at facility in Catania, Italy.

Maryland is the latest state to consider whether to include additional requirements for substitution of biological products

Catalent Pharma Solutions has acquired a license to market Redwood Bioscience 's proprietary SMARTag precision protein-chemical engineering technology.

Dr. Reddy's Laboratories has announced plans to relocate its North America headquarters and establish a R&D facility in Princeton, New Jersey.

FDA Releases Guidance on Self-Selections Studies

Regeneron, a biopharmaceutical company based in New York, has announced plans to expand its corporate headquarters and laboratories in Westchester. This expansion will create more than 400 new highly skilled jobs and further cement Hudson Valley's reputation as an emerging epicenter for biopharmaceutical growth.

Company considers investment in insulin cartridge-filling and insulin API manufacturing capacity in the US.

Teva and Lonza have announced that their joint venture will continue to develop, manufacture and market affordable, efficacious and safe biosimilars.

Company issues voluntary recall after learning of complaints of an uncharacteristic odor coming from Levoxyl bottles.

Astellas and Ambrx have entered into a collaboration to discover and develop novel antibody drug conjugates (ADCs) for an undisclosed number of targets in oncology. ADCs enable targeted delivery of drugs to the target tissue.

USP has inaugurated the first satellite site of the USP Spectral Library Global Laboratory Network in China.

A roundup of additional company and people news from pharmaceutical and biopharmaceutical companies, their suppliers, and contract-service providers.

The Access to Medicines Index shows that pharmaceutical companies are increasing drug development for the developing world and using more tiered pricing mechanisms to lower pricing.

A roundup of developments in corporate social responsibility (CSR) and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations

Latin America's diverse growing market seeks regulatory harmonization.
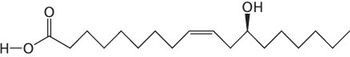
This article summarizes the development and modernization of the United States Pharmacopeia-National Formulary (USP-NF) fixed-oil excipient monographs. This article contains bonus online-exclusive material.

A Q&A with Michael Lacey of the National Institute for Bioprocessing Research and Training

The outlook of the European biotech sector may not be as bleak as apparently believed despite the current economic climate, but compared with their US rivals, biotech companies are still struggling to attract investors and secure funding.