
FDA has issued a draft guidance to assist applicants in the submission of summary-level clinical-site data.

FDA has issued a draft guidance to assist applicants in the submission of summary-level clinical-site data.

Pfizer has agreed to pay USD 55 million plus interest to settle charges that Wyeth (acquired by Pfizer in October 2009) improperly promoted the drug Protonix (pantoprazole).

Amgen Agrees to Acquire deCODE Genetics; Baxter International Agrees to Acquire Gambro; and More.

Roche and the Innovative Medicines Initiative (IMI) have recently launched an academic-industry partnership, known as STEMBANCC, under which 10 pharmaceutical companies and 23 academic institutions will collaborate on using stem cell technology to accelerate the development of safer and more effective drugs for patients.

The US Supreme Court accepted an appeal by the Federal Trade Commission of a decision that upheld an arrangement of payments by Solvay Pharmaceuticals to generic drug companies to postpone introduction of generic versions of its branded testosterone-replacement drug.

Biogen Idec and Isis Pharmaceuticals announced that they have entered into a global collaboration agreement to discover and develop antisense drugs against three undisclosed targets to treat neurological or neuromuscular disorders.

The Court of Justice of the European Union has dismissed an appeal by AstraZeneca concerning a 2005 decision that found the company guilty of abusing its dominant position in the marketplace.

Biogen Idec Opens New RTP Facility; Tekmira, Marina Biotech Enter into License Agreement for UNA Technology; and More.

MeadWestvaco is recognized at CPhI for its sustainable pharmaceutical packaging.

A roundup of developments in corporate social responsibility (CSR) and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

FDA releases guidance documents on PET drugs, labeling of OTC sunscreen, and the use of certain phthalates as excipients.

Myriad Genetics reports that the US Supreme Court has granted certiorari agreeing to hear the company's gene patentability case.

The company issues voluntary recall of certain lots due to possible contamination with small glass particles.

The European Medicines Agency has published a plan to help Europe?s regulatory network prevent, mitigate and manage shortages of important medicines following manufacturing problems.

Eli Lilly released its annual report containing updates on progress and new initiatives in corporate responsibility.

As the battle against counterfeits gains momentum, PharmTech speaks to Mark Davison, CEO of Blue Sphere Health, and Craig Stobie, global life sciences sector manager at Domino Printing Sciences, about the challenges involved.

In addition to globalisation, high financial rewards and low penalties for counterfeiters are contributing to the rise in fake medicines.

Nathan Jessop reviews the pharmaceutical industry's experience with the first year of Germany?s new pricing policy.

A few years ago, drug criminals would put all their efforts into matching packaging and labeling, or manufacturing good-looking fake materials. Today, criminals are capable of much more.

To ensure the accuracy of scientific testing, protect the subjects to avoid data contamination.

A look at the year's leaders in innovation strategy, including the top bio/pharmaceutical companies and award recipients from AAPS, PhRMA, and CPhI.

Domestic companies are changing their business models in response to recent drug price cuts.
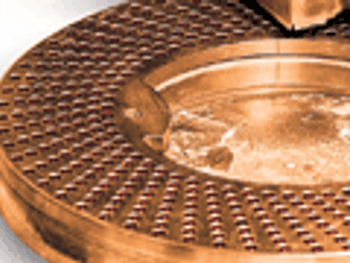
Clean-in-place systems should be optimized during design and commissioning and after validation.

The EMA brought together European and US expert representatives from regulatory authorities, academia, industry and a health-technology-assessment agency on Nov. 18, 2011 to discuss the use and importance of subgroup analysis in the assessment of clinical trials.

Takeda Forms New Global Medical Affairs Department; UCB, NewBridge Pharmaceuticals Partner for Middle East and African Markets; and More.

EMA addresses drug supply shortage.

GlaxoSmithKline announced its intent to increase ownership in its publicly-listed, consumer-healthcare subsidiary in India and in GlaxoSmithKline Consumer Nigeria as part of its emerging-markets investment strategy.

Novartis announced that it has received FDA approval for a seasonal influenza vaccine produced in cell culture, the first seasonal vaccine produced by this method to be approved in the US.

The European Medicines Agency (EMA) recently conducted a workshop on clinical-trial data and transparency in London following the agency's decision to proactively publish all data from clinical trials and enable interested parties to access to full data sets.

The European Commission (EC) is seeking to introduce a black symbol to identify medicines that are subject to additional monitoring.