
Novartis to Construct New Biotechnology Facility in Singapore; Patheon to Acquire Banner Pharmacaps; and More.

Novartis to Construct New Biotechnology Facility in Singapore; Patheon to Acquire Banner Pharmacaps; and More.

Patheon will acquire softgel-specialist company Banner Pharmacaps, based in North Carolina in the US, for $255 million

Novartis is investing more than half a billion US dollars in the construction of a new state-of-the-art biotechnology production site in Singapore for the manufacturing of drugs based on cell culture technology.

AET BioTech and BioXpress Therapeutics have entered into an agreement to codevelop a biosimilar version of Abbott's tumor necrosis factor inhibitor monoclonal antibody adalimumab.

Pharmaceutical industry leaders Novartis and Merck-among others-released second-quarter 2012 results showing global sales down in third-quarter 2012, with growth in key pharmaceutical products helping to offset losses due to patent expirations.

New England Compounding Center gets 483 after linked fungal meningitis outbreak.

Pfizer Announces Intention to Acquire NextWave Pharmaceuticals; Seattle Genetics Expands ADC Collaboration with Abbott; and More.

On Oct. 22, 2012, a consortium in France announced the establishment of Europe's first ever industrial manufacturing facility dedicated to the large-scale production of novel, advanced cell-based therapy medicinal products.

FDA announces Coalition for Accelerating Standards and Therapies and Commissioner Hamburg comments on meningitis outbreak.

Ben Venue Laboratories has resumed production on a limited number of manufacturing lines in the company's Bedford, Ohio, facilities.

Watson Pharmaceuticals has received clearance from the Federal Trade Commission for its acquisition of generic drug manufacturer, Actavis.

Dr Reddy's is planning to acquire the specialty injectable company OctoPlus for approximately EUR 27.4 million ($35.7 million) in cash to strengthen its technological capabilities in drug delivery.

Catalent Licenses NJIT Taste-Masking Technology; MicroConstants Completes FDA Inspection; and More.

EMA releases guideline on medicinal products for the treatment of schizophrenia.

Consider the time and expertise needed to maintain regulatory compliance and perform stability testing.

Teva has invested $110 million in a new sterile plant in Hungary. The company hopes the plant will strengthen the company's role in Hungarian drug development.

New US Pharmacopeial Convention standards provide a universal approach to organizing labels for prescription containers dispensed by US pharmacists in an effort to improve patient understanding.

At this week's AAPS 2012 Annual Meeting in Chicago, graduate students across the country are being honored for their research and work in bio/pharmaceutical innovation. Pharmaceutical Technology had the chance to talk with a few of the recipients.

GlaxoSmithKline has outlined the measures it is taking as part of a new open innovation approach to R&D, including opening up its tuberculosis compound library, investing in its open laboratory in Spain, and sharing detailed clinical trial data with researchers.

Novasep announced that it will be investing EUR 30 million ($39 million) to build what will be the world's largest chromatography plant for the production of large volumes of commercial APIs.

Mayne Pharma to Acquire Metrics; Sanofi, Bristol-Myers Squibb Restructure Plavix Alliance; and More.

Merck has announced plans to close its headquarters in Whitehouse Station, New Jersey, and relocate to its existing facility in Summit, New Jersey, beginning in 2014.

A national fungal meningitis outbreak linked to contaminated vials of steroid injectables from a Massachusetts compounding operation has reignited the debate over the safety of compounded drugs and the need for stronger FDA regulation of these activities. Last week, the Centers for Disease Control and Prevention reported seven deaths and 91 ill.

FDA announced that it is working closely with the Centers for Disease Control (CDC) to identify the source of an outbreak of meningitis among patients who had received an epidural steroid injection.

Takeda's US subsidiary, Takeda America Holdings, has agreed to acquire the vaccine specialist company LigoCyte Pharmaceuticals for an upfront payment of $60 million in a move intended to bolster and expand the company's vaccine business.

Merck Returns Brinavess Rights to Cardiome; Sigma-Aldrich Expands in Scotland; and More.

Merck Serono's Asceneuron is the third company to be spun off from the company's Entrepreneur Partnership Program that was launched to mitigate the impact from recent restructuring at its Geneva site.

Several Big Pharma companies strengthen their manufacturing presence in Russia.
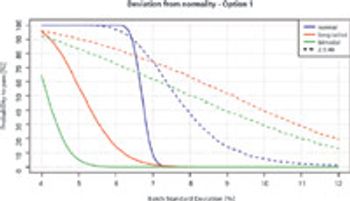
New European Pharmacopoeia chapter aims to resolve problems with applying the harmonized UDU test to large sample sizes.

Will the next US President support the backbone of our industry?