
A subsidiary of Johnson & Johnson initiated a voluntary recall of its antipsychotic medication, Risperdal, due to an unusual odor.

A subsidiary of Johnson & Johnson initiated a voluntary recall of its antipsychotic medication, Risperdal, due to an unusual odor.

Novartis has commenced construction of a new manufacturing plant in Russia that represents the company's most significant investment in the country to date.

Biogen Idec Receives Approval for the Avonex Pen; Xceleron Makes Several Senior Appointments; and More.

GlaxoSmithKline has entered into an agreement to purchase Shenzhen Neptunus? stake in a previously formed joint venture between the companies involved in the development and manufacture of influenza vaccines in China, Hong Kong, and Macau.

FDA Issues Consent Decree of Condemnation, Forfeiture, and Permanent injunction Against H&P Industries, the Triad Group, and Three Individuals.

Merck and Hanwha Chemical have formed an exclusive global agreement to develop and a commercialize a biosimilar of Enbrel, a drug to treat moderate to severe plaque psoriasis, psoriatic arthritis, and moderate to severe rheumatoid arthritis.

An FDA guidance has provided information for products that involve the application of nanotechnology, and rationales for those points.

After looking back at the first year of its Bad Ad outreach program, FDA judged that the initiative has successfully raised awareness about misleading promotion, according to an FDA press release.
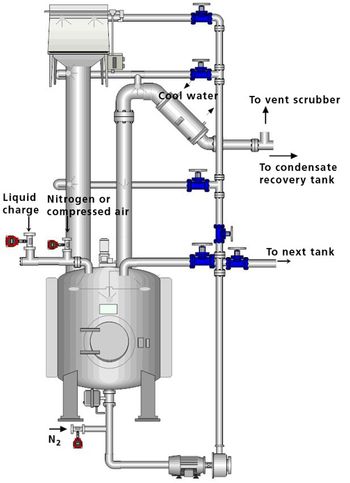
The author describes the benefits and challenges inherent to cleaning in place (CIP). The article also describes the development and validation of a CIP cycle.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the June 2011 edition from Meissner and Telstar.

Our company is getting low and unpredictable cycle life out of our diaphragm valves. This problem has caused us to implement standard operating procedures for frequent replacement of the diaphragms, which is costly and time consuming. What could be happening, and what are our options?

Two popular methods for detecting protein aggregates are analytical ultracentrifugation (AUC) and size-exclusion chromatography?multiangle light scattering (SEC?MALS). These techniques? results correlate relatively well, but each one has its own strengths.

AstraZeneca agreed to settle a sex-discrimination lawsuit by paying $250,000 to 124 women who worked at the company's Philadelphia Business Center.

A South Carolina court found that a subsidiary of Johnson & Johnson violated state consumer protection laws by using misleading marketing for its antipsychotic drug, Risperdal.

The US Supreme Court ruled in favor of Roche in a patent-dispute case the pharmaceutical company had with Stanford University in a 7-2 vote.

Aveo Forms Drug-Development Pact with Centocor Ortho Biotech; Generic Pharmaceutical Association Appoints Jim Fenton as Senior Vice-President of Government Affairs; and More.

The European Public Health Alliance has called for greater pricing transparency, as well as the formation of a public website that provides comparative information on medicines' procurement prices.

FDA Issues Final Guidance to Amend IND Reporting Requirements.

CDER Director Janet Woodcock announces reorganization.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

The World Health Assembly, the decision-making body of the World Health Organization, met in Geneva last month to discuss and take action on initiatives relating to influenza pandemic preparedness, noncommunicable diseases, and the health-related UN Millennium Development Goals.

Eduardo Pisani, director general of the International Federation of Pharmaceutical Manufacturers & Associations, discusses the global health initiatives and related activities of the association.

Can microdosing make medicines safer and more effective for children?

An independent report released by the European Medicines Agency highlighted a number of recommendations to aid the agency in its communication of the benefits and risks of medicines.

Why SOPs are rarely followed, often cited, and in need of follow-through.

A Focus on Creative Strategies to Help the Developing World.

Sometimes, there are just too many cooks in the kitchen.

FDA added a searchable database of inspection data to its website. The database lists the names and addresses of facilities that the agency inspected during fiscal years 2009 and 2010.

FDA weighs multiple views regarding the Biologics Price Competition and Innovation Act.

This month's BIO Convention will encourage needed conversations.