
PhRMA Urges Congress to Reauthorize Legislation for Pediatric Drugs.

The IPEC is soliciting public comment about a draft plan for the independent certification of manufacturers and suppliers of pharmaceutical excipients.

Velesco partners with Vindonwestech; NeurogesX announces retirement of CEO Anthony DiTonno; and More.

A federal appeals court has lifted a ban on federal funding for embryonic stem cell research.

FDA is collecting public comments on a series of studies that the agency plans to conduct on online direct-to-consumer promotion of prescription drug products, according to an announcement in the Federal Register.

Israel-based Teva Industries seals deal to acquire Cephalon for $6.8 billion.

Volume growth in the US prescription drug market was at historically low levels in 2010, and revenue growth was anemic.
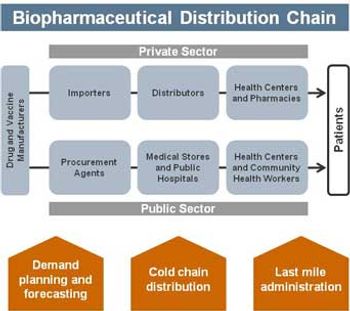
This article, which focuses on distribution and administration, is Part III of a three-part series on biopharmaceutical issues in public health, government, and developing-world markets.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

GlaxoSmithKline and the Singapore Economic Development Board team in advancing sustainable pharmaceutical manufacturing.

As I strolled through Times Square, famous brand coffee cup in hand, towards the Jacob K. Javits Convention Center in New York, my thoughts were entirely focused on Interphex.

Eastern Europe's pharmaceutical leader, Hungary, is working to maintain its number-one status while also pursuing new avenues, especially in biopharmaceuticals.
Efforts are made to educate health workers in less developed countries.

Those who doubt there's faith in science, should check out our annual Bioprocessing Survey.

Can the semiconductor industry help Big Pharma develop therapies?

Monograph modernization and standards donation go hand in hand.

India has the potential to become the new star of the biotechnology industry.

Many factors affect research results.

Despite initiatives to encourage multinational pharma companies to conduct R&D in the UK, the country may be losing its edge; is Pfizer's decision to exit a key site earlier this year the beginning of a mass exodus?

sanofi-aventis signs research pact with Stanford; Paul Maffuid joins AAIPharma Services; and More.

Warner Chilcott announced in a press release on Apr. 18, 2011, its intentions restructure, placing 500 Western European jobs on the line.

The IMS institute has released its report on the use of medicines in the United States during 2010.

Shanghai Pharmaceutical and Pfizer have signed a memorandum of understanding for the companies to jointly pursue potential business opportunities in China.

FDA has released a list of its strategic priorities for the next five years to address new global challenges.

The EMA has concluded a class review of bisphosphonates, adopted a number of positives opinions for new medicines and revealed more about the ongoing studies regarding GlaxoSmithKline's Pandemrix pandemic influenza vaccine and a possible link with narcolepsy.

GlaxoSmithKline (GSK) identified certain over-the-counter (OTC) brands in its consumer healthcare business that the company plans to divest.

The US patent laws are undergoing a major revision, the first large revision since the Patent Act of 1952.

Axcan acquires Mpex Pharmaceuticals; Bend Research receives patent for improving bioavailability of low-solubility drugs; and More.

On Apr. 18, 2011, scientists from the national laboratories of five African nations gathered in Accra, Ghana, to take part in a week of technical training put on by the US Pharmacopeia (USP) that will teach them how to detect substandard and counterfeit medicines.

FDA Released Draft Guidance for Industry on Safety Labeling Changes.