
Company and People Notes: Bayer Schering Pharma forms collaboration with Compugen; Takeda San Francisco appoints VP of process science; more...

Company and People Notes: Bayer Schering Pharma forms collaboration with Compugen; Takeda San Francisco appoints VP of process science; more...

IMS Health delivered some good news last week with its updated pharmaceutical market forecast.

Also, USAID and USP launch counterfeiting public service campaign in Cambodia.

The US Food and Drug Administration issued an alert to healthcare professionals of a change in heparin manufacturing that is expected to decrease the drug's potency.

Also, FDA partners with USDA on produce safety, and the Transparency Task Force announces its second public meeting...

The 2009 Nobel Prize in Physiology or Medicine was awarded to three US researchers for the discovery of how chromosomes are protected from degradation by telomeres and the enzyme telomerase during cell division.

GlaxoSmithKline (London), Novartis (Basel, Switzerland), sanofi Aventis (Paris), and Baxter International (Deerfield, IL) recently provided updates as to the development, manufacture, or shipment of pandemic (H1NI) vaccines.

The US Food and Drug Administration published its first draft guidance for industry about Risk Evaluation and Mitigation Strategies (REMS) on Sept. 30, 2009.

Company and People Notes: GSK forms joint venture with China-based Jiangsu Walvax Biotech; Sigma-Aldrich appoints VP and board member; more...

AAPS President offers hope and solutions for the industry's challenging future.

Pharmaceutical companies in India have had a hold on the biotechnology sector for many years, and they're not about to let the follow-on biologics market pass them by.

BIO supports recent Congressional action toward a 12-year data exclusivity period for innovators.

The nation's healtcare system needs an overhaul, but it has to be done right.

Like life, the workplace also can have many surprises.

Almac's expansion plans in Northern Ireland (UK) could see the creation of more than 500 jobs during the next 5 years.

Contract research organizations such as Covance are heading further east through Europe.
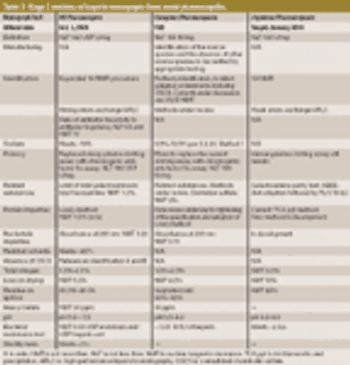
USP workshop participants support new methods to safeguard heparin products but desire international harmonization. This article contains bonus online-exclusive material.

The EMEA intends to fully implement its Product Information Management (PIM) approach, an initiative designed to improve information management, into the centralized procedure within 2 years, according to a statement of intent from the agency.

A review of NIPTE's core projects and its plans for training-and retraining-the pharmaceutical industry.

This article introduces the "Q.U.E.S.T." approach for vendor qualification, a practical and compliant methodology for pharmaceutical and biopharmaceutical companies to qualify vendors and hence make well-informed purchasing-related decisions.

As the pharmaceutical industry moves further into Central and Eastern Europe and the Commonwealth of Independent States, several standard-setting and regulatory bodies are also increasing collaboration in the region, particularly in Russia.

The author describes the framework needed to implement QbD and achieve the deeper process understanding that is fundamental to QbD.

This article is part of a Special Report on the Emerging Markets of The East, October 2009

The author analyzes, from an agency perspective, whether question-based review has improved product quality or made the review process easier for regulators or for industry.

Well, the summer's well and truly behind us and Madrid beckons for this year's CPhI Worldwide.

How manufacturers can accelerate cashflow by cutting out the paper trail.

Also, FDA bans candy and fruit-flavored cigarettes, EMEA moves to improve information management.

A new report evaluating the biotechnology sector in new European Union member states and candidate countries has described biotech developments as "uneven" because of contrasting economic strategies in different countries.

The biopharmaceutical company Seattle Genetics (Bothell, WA) introduced a sugar-engineered antibody (SEA) technology that is designed to increase the potency of monoclonal antibodies through enhanced effector function.

The US Food and Drug Administration issued a proposed rule to clarify the current good manufacturing practice (CGMP) requirements applicable to combination products in the Sept. 23 Federal Register.