
The US Pharmacopeial Convention (USP) and the Permanent Commission of the Pharmacopeia of the United Mexican States (FEUM) signed a memorandum of understanding (MOU) last week.

The US Pharmacopeial Convention (USP) and the Permanent Commission of the Pharmacopeia of the United Mexican States (FEUM) signed a memorandum of understanding (MOU) last week.

Also, Orexo and Novartis form agreement; Affitech appoints Robert Burns CEO; more...

The US Food and Drug Administration last week issued the final draft of its guidance for industry titled Labeling of Nonprescription Human Drug Products Marketed Without an Approved Application as Required by the Dietary Supplement and Nonprescription Drug Consumer Protection Act: Questions and Answers.

Implementing Europe's Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) program, will require a massive increase in animal testing and cost six times more than previously estimated.

Also, FDA issues several recent enforcement letters to Cambrex, Johnson & Johnson, and Pedinol.

An H1N1 vaccine developed by biopharmaceutical company Sinovac Biotech (Beijing, China) has passed the experts evaluation organized by the Chinese State Food and Drug Administration (SFDA) and the company is expected to obtain a production license before the week's end.

Later this month, Dr. Barbara Jentges, the managing director at PhACT GmbH, a regulatory consulting and training firm based in Duggingen, Switzerland, will be speaking at the 2nd Vetter Drug Management Leadership Conference in Germany.

Thanks to their keen observations, these auditors reveal the true culprits of deviations.

After years of promomting QbD concepts, FDA's ready to take action on nonconformers.

An outstanding new book reviews alternative solvents with an eye to sustainable pharmaceutical processes.

A second-generation and green manufacturing process for testosterone provided economic and ecological benefits.
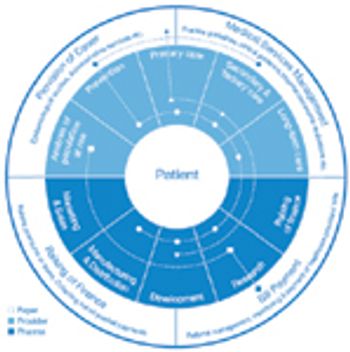
Personalized medicine and integrated healthcare delivery require new business and pricing models. This article contains bonus online-exclusive material.

Representatives of Japan's MHLW report on recent ICH activities and what the ministry expects from Q11.

IPEC's new stability testing guide takes into account the full supply chain's storage conditions.
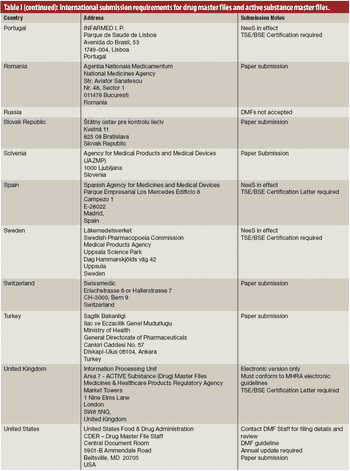
The author describes several issues in creating drug master files and active substance files for active pharmaceutical ingredients and intermediates.

Designated as a "pharmerging market," Brazil is revamping its pricing models.

Jones explains how to save UK pharma.

Merger, union, alliance, partnership, collaboration: five words that ultimately suggest the strengthening of something.

Scientists at Sigma-Aldrich's new Sigma Advanced Genetic Engineering Labs in St Louis (MO, USA) will develop and distribute genetically engineered animal models for use in research.

Eastern Europe's pharmaceutical market is forecast to grow at a CAGR of more than 10% to be worth more than $41 billion by 2014, according to a report from companiesandmarkets.com.

On Aug. 19, 2009, the US Food and Drug Administration opened a new Center for Tobacco Products on the agency's White Oak Campus in Silver Spring, Maryland.

Lonza (Basel) has submitted a non-binding proposal that would see it acquire all of the Restricted Voting Shares of US-based Patheon.

Scientists have modified a tobacco plant to produce a vaccine for norovirus, the viral infection sometimes called the "cruise ship virus."

Researchers at the J. Craig Venter Institute (JCVI), a genomic research organization, reported that they successfully transformed one bacterium into a different strain by transferring the entire bacterial genome of the first strain into a second, related strain of bacteria.

The US Food and Drug Administration announced in an Aug. 20, 2009 release that it would like to amend postmarket safety reporting regulations "to require that manufacturers and other facilities subject to current reporting requirements submit their reports in an electronic format."

Company and People Notes: UCB and Novartis form agreement; AAIPharma appoints VP of regulatory affairs; more...

Also, USP signed a Memorandum of Understanding with the Permanent Commission of the Pharmacopeia of the United Mexican States, more...

A transatlantic strategic partnership has been struck between California (USA) based enterprise software solution provider ValGenesis and IT integration specialists Systec & Services (Germany).

Researchers from a university in the US are urging the FDA to demand that drug manufacturers state how new medications compare with similar treatments on product labels.

Only 43% of European Chief Financial Officers (CFOs) and Chief Information Officers (CIOs) have tried to calculate the impact of outsourcing on their company, and of those that have tried, only 1 in 5 are confident in their sums, according to a study by Cognizant.