
FDA Issues Warning Letter To McNeil Healthcare; Charles River Laboratories Will Suspend Operations At Massachusetts Facility.

FDA Issues Warning Letter To McNeil Healthcare; Charles River Laboratories Will Suspend Operations At Massachusetts Facility.

FDA Thanks Healthcare Workers For H1N1 Response; And More.

The EU's Commissioner-designate for Health and Consumer Policy, John Dalli, said that he is looking forward to the challenge of integrating the pharmaceutical area into public health as part of his role.

McNeil Consumer Healthcare is voluntarily recalling certain lots of over-the-counter products in the Americas, the United Arab Emirates, and Fiji.

Could flexible manufacturing change the standards for biopharmaceutical production? To find out, Equipment and Processing Report talked to James Robinson, vice-president of technical and quality operations at biotechnology company Novavax (Rockville, MD).
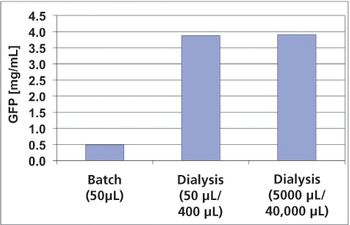
The authors sought to improve the productivity of protein synthesis by using a highly active cell-free extract from Escherichia coli and by optimizing buffer conditions and shaking conditions.

The United States Pharmacopeial Convention is recalling USP 33?NF 28.

Many life-sciences companies are likely to execute an acquisition in the next 12-24 months, according to a survey by Ernst & Young.

The European Commission has asked certain pharmaceutical companies to submit copies of their patent-settlement agreements.

Zydus Cadila, a pharmaceutical company based in Ahmedabad, Gujarat, India, will begin conducting trials for a H1N1 vaccine.

On Jan. 4, 2010, Novartis (Basel) and Nestlé began completing their 2008 agreement, under which Novartis will acquire Nestlé's remaining 52% stake in eye-care company Alcon (Hünenberg, Switzerland).

The US Food and Drug Administration released a warning to the public on Dec. 29, 2009 about criminals posing as FDA special agents and other law enforcement personnel as part of an international extortion scam.

Bioject Medical Technologies establishes alliance with MPI Research; Biogen Idec CEO to retire; And More.

Microelectronics offers "striking opportunities" for advancing biomedical research and creating new markets for the medical sciences industry.
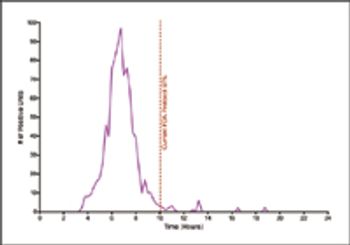
This study used biological indicators containing Geobacillus stearothermophilus spores and a new technology to continuously monitor incubated BIs and record nonsterile results.

A comprehensive book about mass transfer benefits from the author's personal touch.
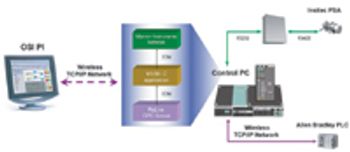
The authors describe the implementation of an on-line particle-size analyzer on an active pharmaceutical ingredient milling operation at a commercial site.
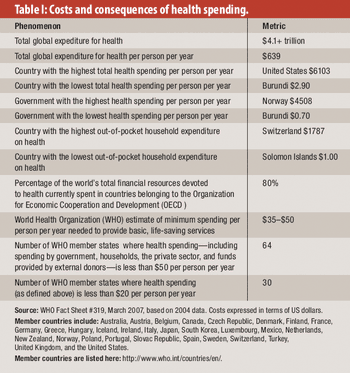
From healthcare to corruption to life expectancy, here's what we can learn from the past decade.

Directors and staff miss the mark when it comes to following procedures.

BioNanomatrix (Philadelphia) names Edward Erickson president and CEO; and More.

Hospira, a specialty pharmaceutical company, has agreed to acquire Orchid Chemicals' Pharmaceuticals for $400 million.

Merck & Co. Acquires Avecia Biologics; Ambrilia Biopharma Closes Manufacturing Facility; And More.

Fraud and abuse in healthcare costs individual governments as much as $23 billion a year, according to estimates from the World Health Organization (WHO).

The European Public Health Alliance published its comments about the Falsified Medicines Directive currently under debate by the European Parliament and Council.

Mergers and acquisitions will contribute almost two-thirds of peer-set sales growth until 2014, according to market analyst Datamonitor.

Eli Lilly outlined its growth strategy at its annual meeting with the investment community last week.

Also, Abbott to acquire Starlims Technologies; GSK Biologicals to form alliance with Intercell.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the December 2009 edition from Sepha and AdvantaPure.

Do advanced therapies represent medical miracles or risky business? This is the question that is debated by an Informa analyst in an Executive Briefing for Scrip.

The European Medicines Agency unveiled several changes to the organization, including a new structure and visual identity.