
Company and People Notes: Also, Pfizer and Protalix enter agreement; Watson acquires Arrow Group; and more.

Company and People Notes: Also, Pfizer and Protalix enter agreement; Watson acquires Arrow Group; and more.

The US Congressional Budget Office released a report that analyzes the pharmaceutical industry?s advertising strategies.

Personalized medicine has been described by analysts PricewaterhouseCoopers as a "disruptive influence" that will create both opportunities and challenges for traditional healthcare.

The private industry has been urged to join a public sector commitment fund in the UK to advance manufacturing R&D in bioprocessing, following the investment of approximately £9 million from two UK research councils.

European business leaders are missing opportunities to reduce their environmental footprint and drive efficiencies through document governance, according to research from Ricoh, an electronic solutions provider.

sanofi-aventis (Paris) announced last week that it has signed a memorandum of understanding (MoU)with Prominvest.

Novartis officially inaugurated its large-scale flu cell-culture vaccine and adjuvant manufacturing facility in Holly Springs, North Carolina.

The US Pharmacopeial Convention posted a proposed revised standard for what should and should not appear on the ferrules and cap overseals of medication vials.

Also, Merck & Co. extends collaboration with Idera Pharmaceuticals; Pfizer establishes R&D center in China; more...

Also, new European Generic medicines Association president begins term.

Pharmaceutical Technology talks to FDA's Justina A. Molzon about current discussions with international regulators, and how, after 20 years of harmonization activities and the common technical document, ICH has benefited regulatory authorities.

Guidance has been published in the United Kingdom regarding a new European variations regulation that comes into effect on Jan. 1, 2010.
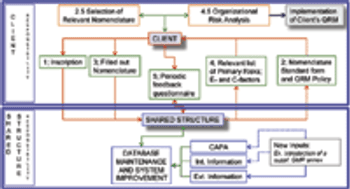
Representatives to the PIC/S provide an example of methodology for implementing ICH Q9.

Readers can learn about the importance of measuring and controlling water activity in a comprehensive new book.

Dumbed-down presentations and poor speaker selections are destroying a valuable industry tool.

With so many healthcare and pharmacy websites, consumers could use the agency's nod of support.

Polymorph screening is a critical step in developing an active pharmaceutical ingredient for a pharmaceutical formulation.

Blame it on cafeteria gossip, outdated procedures, and major miscommunication.
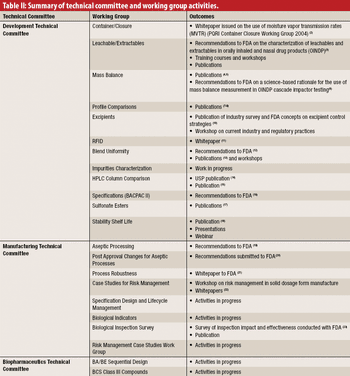
The authors describe the development and organization of the Product Quality Research Institute and highlight some of the important projects conducted by its Working Groups.

It's the end of the year already and I hope you are looking forward to the festive period and the opportunity to wind down and spend some quality time with family and friends.

The end of 2009 is creeping closer and analysts are already looking towards 2010 and wondering what new challenges are in store for the pharma industry.

An agreement between sanofi-aventis and the Medicines for Malaria Venture (MMV) has lead to the initiation of the largest safety and efficacy study of an antimalarial drug.

After further reviewing data on the H1N1 pandemic vaccines approved in Europe, the EMEA has reaffirmed their balance of benefits and risks in the context of the current H1N1 influenza pandemic. Additionally, the World Health Organization (WHO) has issued an update on the H1N1 situation in Europe.

The Society for Chemical Manufacturers and Affiliates (SOCMA) provided an update as to its grassroots efforts regarding the recently passed Chemical and Water Security Act of 2009 (HR 2868).

The US Food and Drug Administration has issued 22 Warning Letters to website operators as part of its International Internet Week of Action.

Company and People Notes: Merck KGaA to build R&D hub in Beijing; Depomed appoints VP of operations; more...

The European Medicines Agency confirmed the safety and efficacy of Focetria and Pandermix, and noted that data on Celvapan are still being analyzed.

Five percent of consumers across five European countries suspect they may have received a counterfeit prescription, and 1% believe that they definitely have, according to research conducted by ICM on behalf of patient safety communications company Aegate (UK).

Engineers at a UK university claim to have developed technology that can be used in existing chemical reactors to ensure right first time drug crystal formation, which is crucial to their efficacy and the efficiency of pharmaceutical manufacturers' operations.

Last week, GlaxoSmithKline (GSK, London) agreed to donate 50 million doses of its adjuvanted pandemic influenza A (H1N1) vaccine to the World Health Organization (WHO).