
Amcor (Australia) has made an offer of $2025 million for parts of Alcan Packaging (France), including the company's global pharmaceuticals division.

Amcor (Australia) has made an offer of $2025 million for parts of Alcan Packaging (France), including the company's global pharmaceuticals division.

Researchers at the University of Illinois (Champaign) have developed a cancer drug delivery system that reportedly kills target tumor cells, spars healthy cells, and has effects that can be reversed to halt potentially hazardous side effects.

Also, FDA publishes draft guidances of two ICH Annexes; EMEA sets format for compliance advice; more...

Researchers from the Stanford University School of Medicine in California are urging the US Food and Drug Administration to demand that drug manufacturers state how new medications compare with similar treatments on product labels.

The US Food and Drug Administration announced a new program regarding the issuance of warning letters to the pharmaceutical industry.

India accounts for approximately 3% of the global outsourcing market, which indicates a significant opportunity for growth, according to a report published by Ernst and Young and the Organization of Pharmaceutical Producers of India.

Only 43% of European chief financial officers (CFOs) and chief information officers (CIOs) have tried to calculate the impact of outsourcing on their company, and of those that have tried, only 1 in 5 are confident about their calculations, according to a study by Cognizant, a global business technology company based in Teaneck, NJ.

An initiative to develop consensus-based industry standards to identify and define green chemicals and process technologies is underway.

Also, XCELERON and GSK form agreement; Millipore appoints VP of life sciences; more...

Manufacturing methods for new drugs could be made greener and more efficient with the help of marine microbes.

The US Food and Drug Administration?s Draft Guidance for Industry?Process Validation: General Principles and Practices provides a life-cycle approach for validating pharmaceutical processes and aims to help pharmaceutical companies achieve consistently high product quality.
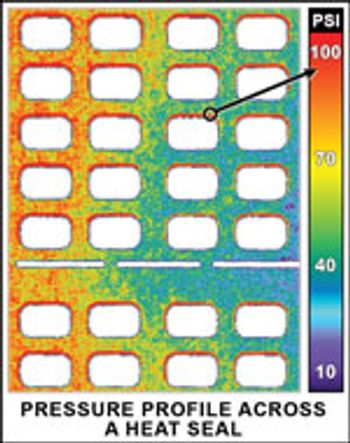
PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the August 2009 edition from AEC and Sensor Products.

Cooling water is a critical component in the research and development, bulk manufacturing, and packaging of pharmaceuticals.

Also: DSM's North Carolina facility receives SafeBridge certification; Dynavax CFO to retire; more...

US Food and Drug Administration Commissioner Margaret Hamburg outlined last week six initial steps designed to improve the effectiveness and timeliness of FDA's regulatory and enforcement system.

Baxter International (Deerfield, IL), sanofi aventis (Paris), and Novartis (Basel, Switzerland) provided updates last week of their production and regulatory activities relating to preparedness in supplying the A(H1N1) pandemic influenza vaccine. Novartis also outlined its activities for providing seasonal flu vaccines.

On August 6, the Biotechnology Industry Organization (BIO) filed an amicus brief asking the US Supreme Court to overturn Bilski v. Doll, a decision of the US Court of Appeals for the Federal Circuit.

The US Food and Drug Administration released its Guidance for Industry titled Pharmaceutical Components at Risk for Melamine Contamination.

Also, EMEA gives public access to GMP data; SOCMA speaks out on toxic substances; more...

An agreement has been reached between Pfizer and Nigeria's Kano State government following the long-running Trovan trial, which stems from the accusation that Pfizer illegally tested the antibiotic Trovan (trovafloxacin) on children in 1996 during a meningitis outbreak.

The Society of Chemical Manufacturers and Affiliates (SOCMA) reported in late July that Eurostat, the Statistical Office of the European Communities, recently published a baseline study or the first snapshot of REACH policy in the preregistration phase.

The US Food and Drug Administration announced its prescription drug user fee rates for fiscal year 2010 in the August 3 Federal Register.

Also, Pfizer forms two research agreements in China; NanoInk appoints John Kubricky to its scientific advisory board; more...

The Energy and Commerce Committee of the US House of Representatives approved H.R. 3200, America's Affordable Health Choices Act, last Friday.

The US Food and Drug Administration and European Medicines Agency (EMEA) launched this week a joint initiative to collaborate on international good clinical practice (GCP) inspection activities.

Novartis has confirmed that the ashes of CEO Daniel Vasella's mother have been stolen and his holiday home set fire to.

South Africa was the first country on the African continent to become a PIC/S member. The country's Director of Inspectorate and Law Enforcement describes the 10-year process.

Big Pharma entered biotech too late. That same mistake can be avoided in personalized medicine.

The author of an ambitious book about quality control falls short of reaching his goals.

The author of a book about biopharmaceutical production includes irrelevant information.