
A new guidance issued by the US Food and Drug Administration earlier this month advises companies on how to treat polymorphic drug compounds?those that exhibit multiple structural forms?in filing abbreviated new drug applications.

A new guidance issued by the US Food and Drug Administration earlier this month advises companies on how to treat polymorphic drug compounds?those that exhibit multiple structural forms?in filing abbreviated new drug applications.

Albany Molecular Research acquires manufacturing sites, Bespak restructures, more

Here's a look at some of the most interesting and thematic responses you-our readers-provided for Pharmaceutical Technology's anniversary survey of industry advances and directions.

The good ol' days weren't always good.

Thirty years ago, the world was a very different place; so was the pharma industry.
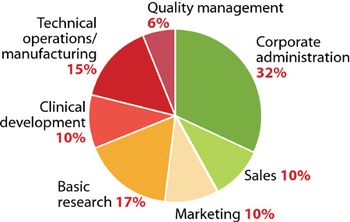
Trenton, NJ (May 18)-Pharmaceutical and medical technology companies in New Jersey have found a striking disparity between six high-demand occupations and the number of qualified workers to fill those positions, according to a report issued by the HealthCare Institute of New Jersey (HINJ). Modest job growth in this field is expected for the next four years, states the report.

Akorn, Catalent, Novo Nordisk, more

Washington, DC (June 22)-Senators Orrin Hatch (R-UT), Edward Kennedy (D-MA), Michael Enzi (R-WY), and Hillary Clinton (D-NY) agreed on legislation that would authorize the US Food and Drug Administration (Rockville, MD) to approve follow-on versions of biologic therapies.

June 21 (London)-The European Medicines Agency agreed to follow up with patients affected by Roche Registration Limited's (Basel, Switzerland) "Viracept" (nelfinavir), which was recalled earlier this month. The drug, an antiretroviral medicine used to treat HIV-1 infected patients older than three years, was recalled from the European market after it was learned that some batches had become contaminated with ethyl mesilate during manufacturing.

London, UK (June 22)-The competition between biosimilars and branded drugs intensified this week thanks to favorable reviews of three biosimilar products for a popular treatment for anemia.

Double-digit growth is projected for the US generic drug market, and the industry positions for opportunities in biosimilars.

Althea Technologies partners with GeoVax Labs, Nabi Biopharmaceuticals restructures, more

Rockville, MD (June 18)-The US Food and Drug Administration, the European Commission, and the European Medicines Agency have agreed to extend cooperative activities to the areas of pediatrics and medicinal products for rare diseases (i.e., orphan drugs).

New York (June 19)-The US District Court for the Southern District of New York affirmed the validity and enforceability of Sanofi-Aventis's patent on clopidogrel bisulfate, the active ingredient in the company's coronary artery disease treatment "Plavix."

Washington, DC (June 14)-The US Department of Health and Human Services awarded two contracts totaling $132.5 million to Sanofi Pasteur and MedImmune to retrofit their influenza vaccine manufacturing facilities.

Basel, Switzerland (June 6)-Roche, in an agreement with the European Medicines Agency and the Swiss Agency for Therapeutic Products, recalled all batches of "Viracept" (nelfinavir) powder and tablets in Europe and some other regions of the world.

Cambridge, MA (June 7)-Genzyme Corp. is growing, with a new biomanufacturing plant planned and other expansions nearing completion.

Los Angeles (June 6)-Researchers at the University of California at Los Angeles (UCLA) used silica-based nanoparticles to deliver the anticancer drug camptothecin (CPT) and other water-insoluble drugs to human cancer cells.

Brussels (May 30)-The European Federation of Pharmaceutical Industries and Associations (EFPIA) called for the pan-European and industry-wide adoption of 2-D barcode technology to combat the increase in counterfeit drugs in Europe.


Kvistgard, Denmark (June 4)-Bavarian Nordic received a $1.6-billion contract from the US Department of Health and Human Services to manufacture and deliver 20 million doses of its smallpox vaccine "Imvamune."

AstraZeneca, Barr Pharmaceuticals, Jubilant Organosys, Roche, more

Thousand Oaks, CA (June 4)-Amgen agreed to acquire Ilypsa (Santa Clara, CA), a private company that develops nonabsorbed drugs for renal disorders, for $420 million.

Rockville, MD (May 31)-The US Food and Drug Administration issued a draft guidance on how to design bioequivalence (BE) studies for specific drug products to support abbreviated new drug applications (ANDAs).

Rockville, MD (June 4)-Americans may soon have access to more information about the risks and benefits of the prescription and over-the-counter drugs they take. The US Food and Drug Administration is establishing an advisory committee to counsel the agency on how to strengthen the communication of risks and benefits of FDA-regulated products to the public.

Rockville, MD (May 31)-The US Food and Drug Administration finalized guidances for seasonal and pandemic influenza vaccines, outlining the regulatory pathways for developing and approving these products.

Look ahead to keep from falling behind.

Global corporate citizenship can go a long way.

Perhaps Congress can help FDA meet its multiple oversight demands.

Signature authentication technology has evolved to become a stong tool for improving confidence in verification.