
Our files pull up lost contracts, poor competitive tactics, and an over-ambitious employee.

Our files pull up lost contracts, poor competitive tactics, and an over-ambitious employee.

Courts are denying the terminally ill access to experimental drugs, leaving many with no options.

Company and People Notes: Evotec and Renovis enter agreement, Amgen to lay off 675 workers, more.

The European Medicines Agency?s Committee for Medicinal Products for Human Use recommended the lifting of the suspension of the marketing authorization for ?Viracept? (nelfinavir mesylate) from Roche and the re-introduction of the drug in the European Union.

Reflecting a strategic interest to strengthen its position in biologics, Bristol-Myers Squibb agreed to acquire the biopharmaceutical company Adnexus Therapeutics for $430 million.

Rep. John D. Dingell (D-MI), chairman of the Committee on Energy and Commerce, along with Reps. Frank Pallone (D-MI), chairman of the Subcommittee on Health, and Bart Stupak (D-MI), chairman of the Subcommittee on Oversight and Investigations, introduced legislation that would create a user fee on imported drug and food shipments.

The US Food and Drug Administration has denied shipments of active pharmaceutical ingredients manufactured at a production facility of Kunshan Chemical and Pharmaceutical Co. for violation of good manufacturing practices, according to an FDA warning letter issued Sept. 6, 2007.

House and Senate leaders finally agreed on compromise legislation to renew prescription user fees, just a few days before the funding program was set to expire.

Company and People Notes: Catalent expands Bolton, UK, warehouse; Biotica appoints Edward E. Hodgkin as CEO and director; more.

Pfizer plans to cease all remaining manufacturing operations at its facility in Sandwich, Kent, United Kingdom. The closure will result in the loss of approximately 420 jobs, phased over the next two years, according to a company release

Bayer Schering Pharma officially completed the acquisition of Novartis?s biologics manufacturing facility in Emeryville, California.

US Food and Drug Administration Commissioner Andrew von Eschenbach warned agency employees last Friday that 2,000 layoff notices could be coming as early as Sept. 21.

454 Life Sciences, part of Roche, and researchers at Columbia University identified a virus associated with the deaths of 2.4 million honey bee colonies.

Company and People Notes: Baxter and Halozyme Expand Relationship, Crucell Names COO, More.

Senators Chuck Grassley (R-IA) and Herb Kohl (D-WI) introduced the Physician Payments Sunshine Act to require pharmaceutical and biologics manufacturers to report the payments, gifts, honoraria, and trips they give to doctors.

More than 100 people attended this week’s Regulatory Affairs Conference by the International Pharmaceutical Excipients Council of the Americas to discuss the latest trends and challenges in the excipient supply chain.

The US Food and Drug Administration’s Interagency Working Group on Import Safety released a Strategic Framework based on a cost-effective, risk-based approach for ensuring the safety of products exported to the United States.

The United States House of Representatives passed its version of the Patent Reform Act of 2007 (H.R. 1908) in a 220–175 vote.

The US Food and Drug Administration issued a final guidance, Manufacturing Biological Intermediates and Biological Drug Substances Using Spore-Forming Microorganisms, which provides recommendations that allow for greater flexibility when manufacturing biological products with spore-formers.

Early this month, Congress is expected to debate what some companies are calling the most significant changes to the United States patent system in 50 years.

A team of researchers from the University of Idaho and Seoul National University have designed a potential drug delivery system by coating silica nanowires with the protein fibronectin.

The US Food and Drug Administration issued a draft guidance, Pharmacogenomic Data Submissions Companion Guidance to be used as a companion to an earlier guidance, Pharmacogenomic Data Submissions, which was issued in March 2005.

Ranbaxy Laboratories has gained approvals from the US Food and Drug Administation to manufacture and market galantamine hydrobromide tablets and carvedilol tablets.

Company and People Notes: SAFC Expands in Wisconsin, Amgen Reduces Rhode Island Staff, More.

Boehringer Ingelheim decided to voluntarily withdraw its “Silomat” drug, which contains clobutinol hydrochloride, in all countries where it is available.
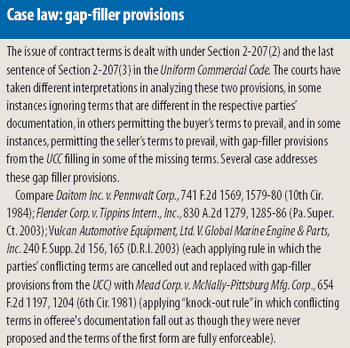
Purchase-order contracting is a commonly used approach to conducting commercial transactions, but it is a risky proposition when applied to pharmaceutical transactions, including the buying and selling of contract services and pharmaceutical ingredients. The authors examine the contract provisions covered in a commercial-supply agreement that are likely to be omitted under purchase-order contracting and the risk-reduction benefits that a commercial-supply agreement can offer in pharmaceutical procurement transactions.

Aptuit expands its drug-development capabilities with the formation of Aptuit Laurus to take advantage of the growing pharmaceutical outsourcing market in India.

FDA must increase inspections of foreign API manufacturing facilities as more production moves offshore.

Poor processing and misguided projections lead to trashed product.

In an age of "pre-existing conditions," can a new bill help patients get the treatment they need?