
Mergers and acquisitions have changed the shape of the contract services market as big players seek to build full-service capabilities.

Mergers and acquisitions have changed the shape of the contract services market as big players seek to build full-service capabilities.

Only half of new drugs make it past Phase III. Adopting best practices and collaborating more closely with contract research organizations can help ensure success.

The partners will collaborate on a project to capture and integrate clinical trial data metrics on investigational cancer medications.

EMA confirms its recommendation to suspend medicines approved based on GVK Biosciences studies.

Pfizer announces that its Phase III trial for Ibrance met its primary endpoint and was ended early due to efficacy based on an assessment by an independent Data Monitoring Committee.

EMA Recommends Suspension of 700 Drugs Tested at GVK Site GVK Biosciences argues that EMA’s recommended suspension of 700 drugs is disproportional to reported infractions. The European Medicines Agency (EMA) has issued a recommendation that 700 medicines authorized for use in the European Union (EU) should be suspended, based on concerns about how GVK Biosciences, a contract research organization in Hyderabad, India, conducted clinical studies. GVK Biosciences, in response, argued “the action is unprecedented and highly disproportional.”

Evolving clinical trial research services give biopharmaceutical companies options for full and functional services.

Geographic location, customs and drug-development regulations knowledge, and transportation infrastructure were factors in Catalent Pharma Solutions' selection of Shanghai for its clinical-trials supply facility.

The relatively low success rates for bio/pharmaceutical compounds in clinical development have prompted many organizations to explore ways of reducing risk in clinical development.

Clinical research organizations see reform in clinical-trial process, including the establishment of chief innovation officer at FDA.

The authors examine risk management relating to the quality issues of clinical-trial materials and discuss areas that would benefit from additional consideration and harmonization.

The author analyzes the results of a survey that polled pharmaceutical executives and managers about both sides of the outsourcing relationship. Read this and other preferred organization articles in this special issue.

The FDA has issued a final rule that clarifies what safety information must be reported during clinical trials of investigational drugs and biologics.

As emerging markets become increasingly important for the pharmaceutical majors, companies are re-evaluating their outsourcing strategies. This article is part of the 2010 Outsourcing Resources special issue.

Pfizer Ends Second Tanezumab Clinical Program; Catalent VP Joins USP Panel; And More.

From product development to finished patient kits, Rockwell Automation equipment is used throughout the entire process.

The author examines the use and advantages of disposable technologies in the fill–finish of sterile pharmaceutical products and how these technologies can reduce costs and time in producing clinical-trial materials.

CROs and CMOs expand to gain a piece of the market for clinical trial materials.
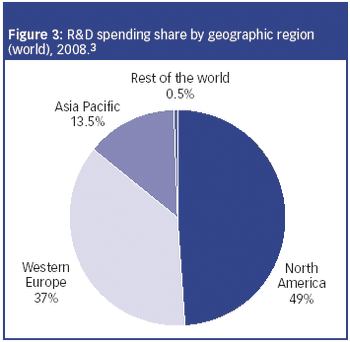
The CRO market is experiencing two-tiered growth: firstly from pharmaceutical companies seeking to lower fixed costs by outsourcing clinical research to CROs; and secondly, from biotechnology and specialty pharmaceutical companies that lack the infrastructure to conduct trials.

CMC service providers are doing well, but clinical and preclinical CROs are doing even better.
The outsourcing of clinical-trial materials grows as pharmaceutical companies adapt to a changing market.

The authors evaluate the scalability of foam-granulation technology using continuous foam addition in high-shear granulation equipment at the laboratory, pilot and manufacturing scales. Immediate- and controlled-release model formulations were used. Continuous and batch addition of foam were compared for the controlled-release model formulation at the manufacturing scale, and physical testing was performed on the granules and finished tablets.

This article is written to assist clinical manufacturing representatives at pharmaceutical companies who are faced for the first time with outsourcing the manufacture of clinical supplies. The author describes the identification, writing, and execution of documents required to support the contract manufacture of products for clinical studies.