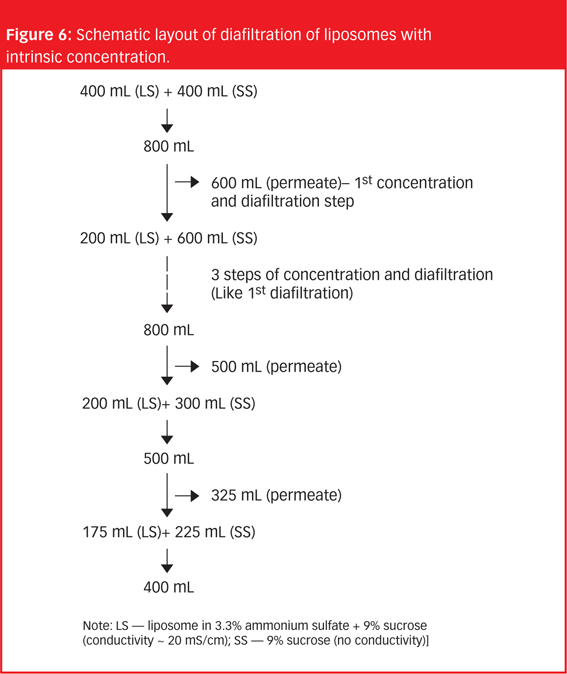
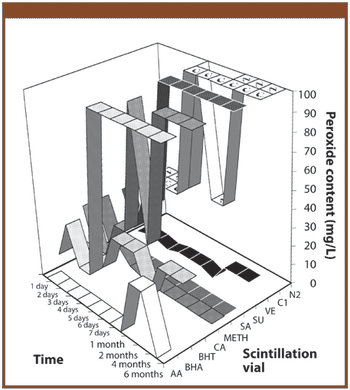
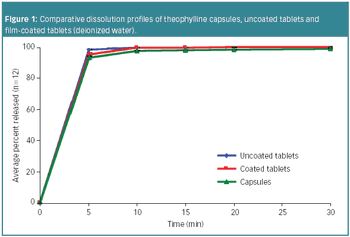
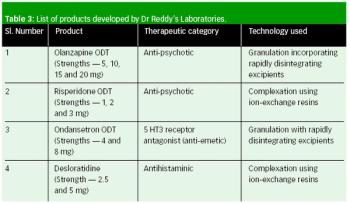
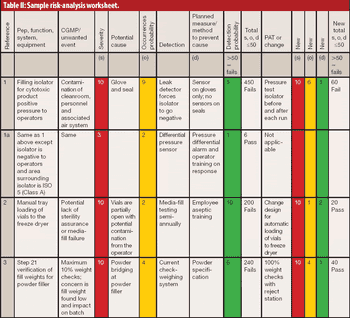
In 2005, a small delegation (myself included) of the European Fine Chemicals Group (EFCG) met with the deputy head of the cabinet of Commissioner Kyprianou (the then Commissioner responsible for health and consumer protection). Our mission was simple - we were there to raise a red flag.