
The authors relay the outcome of a two-day workshop that brought together regulators and generic-drug industry representatives.

The authors relay the outcome of a two-day workshop that brought together regulators and generic-drug industry representatives.

The nation's healtcare system needs an overhaul, but it has to be done right.

Like life, the workplace also can have many surprises.

New nanotechnology-based delivery systems offer promise in drug delivery, particularly for anticancer therapeutics.

Brief pharmaceutical news items for October 2009.

Representatives of one pilot program participant, Wyeth, outline the experiences and lessons learned for implementing a science- and risk-based approach to drug-development and manufacturing.

The author discusses control strategies via near infrared instrumentation for continuous mixing, granulation, drying, and extrusion with a more focused detail on mixing.

Officials from the US Food and Drug Administration discuss best practices for applying quality-by-design concepts. This article contains bonus online-exclusive material.
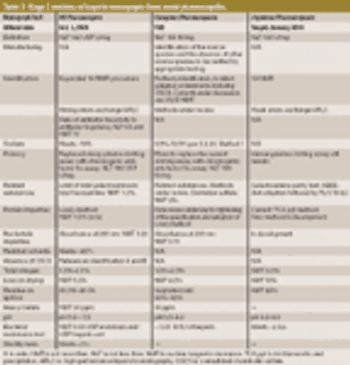
USP workshop participants support new methods to safeguard heparin products but desire international harmonization. This article contains bonus online-exclusive material.

A review of NIPTE's core projects and its plans for training-and retraining-the pharmaceutical industry.

The FDA issued a proposed rule to clarify the cGMP requirements applicable to combination products in the Federal Register.

The author describes the framework needed to implement QbD and achieve the deeper process understanding that is fundamental to QbD.

Endotoxin removal from a finished product is a major challenge for biopharmaceutical manufacturers; particularly as all endotoxin removal methods have operational limitations and may result in loss of protein.

Formoterol presents formulators and manufacturers in the asthma and chronic obstructive pulmonary disease marketplace many challenges.

Company and People Notes: Boehringer Ingelheim will acquire Wyeth's animal health business; Amsterdam Molecular Therapeutics appoints CEO; more...

Company and People Notes: Wyeth and Ambrx form development pact; Elite Pharma appoints CEO and CSO; more...

Company and People Notes: Sanofi Pasteur signs H1N1 vaccine deal with Brazilian government; Helsinn Group appoints CEO of US business; more...

Generic-drug companies are increasingly viewing the development of controlled-release formulations as a way of obtaining a competitive edge, according to a report published by Espicom Business Intelligence in August 2009.

Company and People Notes: Neoprobe and Laureate Pharma form manufacturing agreement; Akela Pharma appoints CEO and chairman; more...

The US Pharmacopeial Convention (USP) and the Permanent Commission of the Pharmacopeia of the United Mexican States (FEUM) signed a memorandum of understanding (MOU) last week.

Also, Orexo and Novartis form agreement; Affitech appoints Robert Burns CEO; more...

Brief pharmaceutical news items for September 2009.

Thanks to their keen observations, these auditors reveal the true culprits of deviations.

After years of promomting QbD concepts, FDA's ready to take action on nonconformers.

Select contract manufacturing organizations roll out expansions for production of active pharmaceutical ingredients and intermediates.