
Conventional tablets may no longer be the go-to solution.

Conventional tablets may no longer be the go-to solution.

Innovations in drug delivery such as needle-free transfer devices and vial adapters can provide consistency when transferring liquid for reconstitution of lyophilized drug products.
Advances in engineered particles and the subsequent reduction in the API mass required to achieve a therapeutic dose can lead to a reduction in side effects.
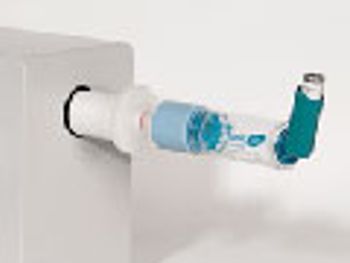
The role of add-on devices and how they affect drug delivery with a pressurized metered dose inhaler.

A surge in generic drug applications is testing FDA?s ability to reduce the backlog of ANDAs.

The National Institute of Allergy and Infectious Diseases will begin the first in a series of trials for an Ebola vaccine during the week of Sept. 1.

Concerns about quality in overseas production may bring generic drug production back to the United States, concludes CPhI study.

The European system for assessing drugs will be used as a model internationally.

EMA has released the second module of a new guideline on influenza vaccines for a six-month public consultation. The guidance covers the non-clinical and clinical requirements for the development of new influenza vaccines and aims to facilitate the prompt assessment of new vaccines. It follows the publication of a module on the quality requirements.

Customers are looking to reduce risk, increase performance, and optimize productivity.

FDA seeks high quality applications and products to facilitate approvals and reduce safety and supply problems.

Experts from Capsugel and Catalent discuss the rationale of using lipid0basd formulations to improve the oral bioavailability of poorly soluble drugs.

New chemical entities with poor aqueous solubility require the use of technologies to enable sufficient oral bioavailability of these NCEs following administration.

With a number of branded biologics hanging at the patent cliff, the future looks promising for biosimilars.

Experts discuss developments and future trends in prefilled syringes.

To realise the full potential for biosimilars, stakeholders must build a better understanding of biosimilars and to take a comprehensive view of how these important medicines can fit into treatment pathways.

Stephen Tindal from Catalent Pharma Solutions speaks with Pharmaceutical Technology Europe about advances in softgel technologies.

Softgels are now being recognised as one of the preferred dosage forms for the treatment of pain, eye conditions, cough and cold, as well as allergy.

Contract research, development, and manufacturing organizations invested in new facilities and technologies in 2014 for analytical testing, as well as small- and large-molecule drug development.

Representatives of contract service organizations that develop biologic-based drugs discussed technology trends such as high-throughput screening and single-use systems.

Pfizer announced an agreement to acquire Baxter International?s marketed vaccines for $635 million.

Redbiotec and GE Healthcare Life Sciences develop a novel manufacturing process for virus-like particles.

The regulatory filing marks the first step in the approval process toward making RTS,S available as an addition to existing tools currently recommended for malaria prevention.

Study provides first substantive reference data on key quality attributes of empty capsules

Sandoz is first to file for FDA approval of a biologic under the biosimilars pathway created in the Biologics Price Competition and Innovation Act of 2009.