
The ongoing battle over drug reimbursement and pricing has raised questions about whether the pharmaceutical industry can continue to rely on high United States revenues to fund biopharmaceutical R&D.

The ongoing battle over drug reimbursement and pricing has raised questions about whether the pharmaceutical industry can continue to rely on high United States revenues to fund biopharmaceutical R&D.

The Center for Drug Evaluation and Research seeks a more flexible system for assessing biotech product quality.

Pfenex announced that it entered into an agreement to partner with Hospira on the development and commercialization of PF582, a biosimilar candidate to Lucentis.

Amgen announced that it met primary and secondary endpoints in its biosimilar evaluation of adalimumab for the treatment of rheumatoid arthritis, when compared to Humira.

Sanofi launched a rapidly absorbed, short-acting inhalable insulin in the US to help control type 1 and type 2 diabetes.

Mylan announced a partnership with Theravance Biopharma to develop and commercialize TD-4208, a novel investigational COPD treatment.

The 2016 White House Budget proposes a change to the data exclusivity period for biologics and the authority to influence drug pricing.
Ligand-binding assays are fundamental to characterizing biosimilars.

EMA is under pressure to exert even tighter standards on biosimilars being marketed in Europe.

Market forces may limit the success of CMOs.


The authors discuss a novel particle engineering technology based on mechano-chemical activation.
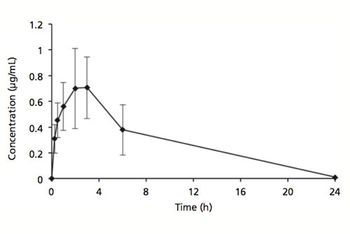
Formulating an injectable solution containing both hydrophilic and hydrophobic drugs is a challenge.
Working with biological matrices and understanding the intended use are crucial.

New guidelines focused on the materials of construction in biologic therapy packaging will help vendors prepare comprehensive extractable and leachable testing strategies.

CMO executives share their opinions on where outsourcing is going and what is driving market change.
After launching a new mammalian cell platform, FUJIFILM Diosynth Biotechnologies U.S.A., has acquired fast-track vaccine manufacturing knowhow and a major presence in Texas’ emerging biocorridor with Kalon, its first acquisition.

Catalent will add potent containment for blending and granulation at its New Jersey manufacturing Center of Excellence.

Government and industry efforts to address manufacturing challenges move Ebola vaccine candidates into larger clinical trials.

A draft NIOSH Current Intelligence Bulletin on recommendations for reducing worker exposure to reproductive risks of drugs is available for public comment.

Catalent announced that it would partner with Mitsubishi Gas Chemical Company, and its subsidiary MGC Pharma, to promote GPEx technology, a high-titer vector for stable mammalian cell lines.

Sanofi has entered into a strategic manufacturing collaboration with Boehringer Ingelheim for the manufacture of therapeutic monoclonal antibodies (mAbs).

Catalent Pharma Solutions and Sanofi-Aventis R&D have entered into a collaboration to develop Sanofi’s proprietary antibodies using Catalent’s SMARTag antibody drug conjugate (ADC) platform.

Mexichem announced that it acquired the distribution and sale license for HFC-227ea/P from Du Pont.

An FDA expert committee has recommend approval of Zarizio, the first US biosimilar application from Sandoz, setting a milestone for generic biologic drugs and setting the stage for future approvals.