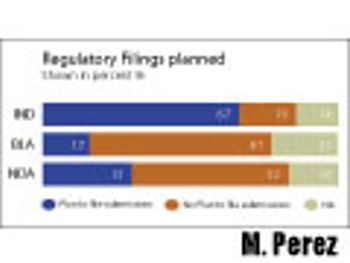
How to cut time and cost by re-using already submitted documents.

How to cut time and cost by re-using already submitted documents.

Novartis CEO To Step Down; DSM To Close Netherlands Facility; And More.

Lonza Group (Basel) plans to reinforce its presence in Asia, including its platform in Nansha, China, and to close its Conshohocken (Riverside), Pennsylvania; Shawinigan, Canada; and Wokingham, United Kingdom, sites in 2010.

FDA Issues Warning Letter To McNeil Healthcare; Charles River Laboratories Will Suspend Operations At Massachusetts Facility.

The United States Pharmacopeial Convention is recalling USP 33?NF 28.

Bioject Medical Technologies establishes alliance with MPI Research; Biogen Idec CEO to retire; And More.

Team leaders in FDA's Office of Generic Drugs provide an overview.
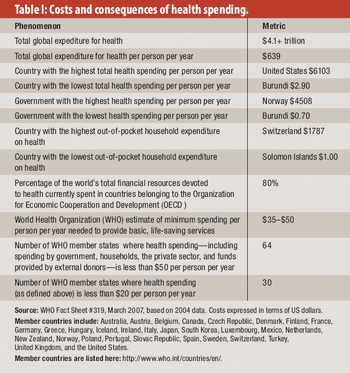
From healthcare to corruption to life expectancy, here's what we can learn from the past decade.

Directors and staff miss the mark when it comes to following procedures.

BioNanomatrix (Philadelphia) names Edward Erickson president and CEO; and More.

Merck & Co. Acquires Avecia Biologics; Ambrilia Biopharma Closes Manufacturing Facility; And More.

Eli Lilly outlined its growth strategy at its annual meeting with the investment community last week.

Also, Abbott to acquire Starlims Technologies; GSK Biologicals to form alliance with Intercell.

Company and People Notes: Also, Pfizer and Protalix enter agreement; Watson acquires Arrow Group; and more.

Also, Merck & Co. extends collaboration with Idera Pharmaceuticals; Pfizer establishes R&D center in China; more...
In this article, the authors evaluated the effects of the granulating binder level, binder type, water amount, and water-droplet size on the MADG process.

Particularly in a more connected world, individual contributions can make a difference.

Dumbed-down presentations and poor speaker selections are destroying a valuable industry tool.

With so many healthcare and pharmacy websites, consumers could use the agency's nod of support.
Leading experts share insight on polymorphism and crystallization.

Polymorph screening is a critical step in developing an active pharmaceutical ingredient for a pharmaceutical formulation.

The authors discuss a study that demonstrates the use of polyethylene oxide mixtures to investigate the effect of polymer viscosity on formulation robustness.

The Society for Chemical Manufacturers and Affiliates (SOCMA) provided an update as to its grassroots efforts regarding the recently passed Chemical and Water Security Act of 2009 (HR 2868).

Company and People Notes: Merck KGaA to build R&D hub in Beijing; Depomed appoints VP of operations; more...

Company and People Notes: Genzyme provides updates on enzyme replacement products and FDA complete response letter; Watson appoints VP of global operations; more...