
Company and People Notes: Neoprobe and Laureate Pharma form manufacturing agreement; Akela Pharma appoints CEO and chairman; more...

Company and People Notes: Neoprobe and Laureate Pharma form manufacturing agreement; Akela Pharma appoints CEO and chairman; more...

The US Pharmacopeial Convention (USP) and the Permanent Commission of the Pharmacopeia of the United Mexican States (FEUM) signed a memorandum of understanding (MOU) last week.

Also, Orexo and Novartis form agreement; Affitech appoints Robert Burns CEO; more...

Brief pharmaceutical news items for September 2009.

After years of promomting QbD concepts, FDA's ready to take action on nonconformers.

An outstanding new book reviews alternative solvents with an eye to sustainable pharmaceutical processes.

A second-generation and green manufacturing process for testosterone provided economic and ecological benefits.

Select contract manufacturing organizations roll out expansions for production of active pharmaceutical ingredients and intermediates.

Pfizer uses green-chemistry in a second-generation manufacturing route for gabapentin.

As new process validation guidelines emerge, industry needs to reinvent how it releases product.

IPEC's new stability testing guide takes into account the full supply chain's storage conditions.
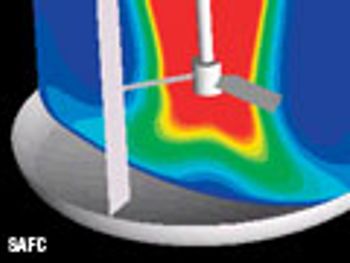
The authors discuss the advantages of microreactors and flow chemistry for various reaction types in achieving improved process economics and reaction efficiency.
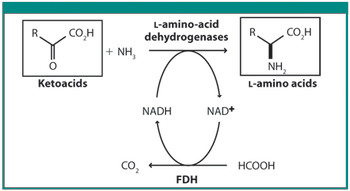
Scientists from DSM and Kaneka discuss various techniques in this roundtable moderated by Patricia Van Arnum.

The authors describe the various available technologies used in orally disintegrating tablets.

Company and People Notes: UCB and Novartis form agreement; AAIPharma appoints VP of regulatory affairs; more...

Also, FDA publishes draft guidances of two ICH Annexes; EMEA sets format for compliance advice; more...

An initiative to develop consensus-based industry standards to identify and define green chemicals and process technologies is underway.

Also, XCELERON and GSK form agreement; Millipore appoints VP of life sciences; more...

Manufacturing methods for new drugs could be made greener and more efficient with the help of marine microbes.

Also: DSM's North Carolina facility receives SafeBridge certification; Dynavax CFO to retire; more...

Baxter International (Deerfield, IL), sanofi aventis (Paris), and Novartis (Basel, Switzerland) provided updates last week of their production and regulatory activities relating to preparedness in supplying the A(H1N1) pandemic influenza vaccine. Novartis also outlined its activities for providing seasonal flu vaccines.

The Society of Chemical Manufacturers and Affiliates (SOCMA) reported in late July that Eurostat, the Statistical Office of the European Communities, recently published a baseline study or the first snapshot of REACH policy in the preregistration phase.

Also, Pfizer forms two research agreements in China; NanoInk appoints John Kubricky to its scientific advisory board; more...
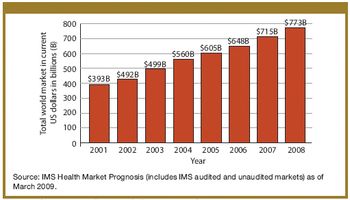
Biologics enhance their positions amidst slowing growth in the global and US markets.

Brief pharmaceutical news items for August 2009.