
Thermo Fisher Scientific is increasing access to cry-electron microscopy with the help of contract research organizations.

Thermo Fisher Scientific is increasing access to cry-electron microscopy with the help of contract research organizations.

The award will help a bioanalytics startup commercialize instrument technology for pharmaceutical antibody manufacturers.

The use of analytical assays is crucial for determining that biosimilar critical quality attributes remain on point.

The investment will allow for the expansion of analytical R&D efforts and will support the company’s reference materials business.

The company’s SLIM technology has been a part of the company’s accelerated growth by offering enhanced R&D analytics.

The company has launched a residual GMP-compliant test and dual sourcing opportunity designed to increase compliance and reduce risks for ATMP manufacture.

The company has begun development of a 3D bioreactor for the cGMP production of extracellular vesicle exosomes.

Stevanato Group and Colanar have signed an agreement for lab scale fill/finish capabilities to study container closure systems at Stevanato Group’s US TEC in Boston, MA.
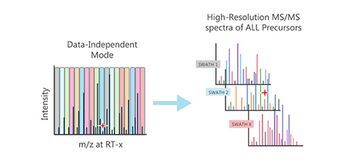
This method is expected to help bring gene therapies to market faster, safer, and cheaper.

The new SDMS automatically gathers and protects all instrument and other laboratory data as it is generated for enhanced lab management, quality, and security.
Appropriate analytical assays are needed to determine and ensure that biosimilar critical quality parameters are on track.

Enhanced sensitivity and ultra-high detection speed provide improved robustness and operability.

Pall has introduced a 24-well filter plate that can perform cell clarification and sterilization in one step.

The company has provided updates on its COVID-19 tests under development with Adeptrix and Cytiva, respectively.

The company has expanded its laboratory test portfolio with five new molecular, serology, and functional assays for COVID-19 vaccine and therapy development programs.

Recent drug recalls in the United States from nitrosamine contamination prompt the need for more sensitive impurity testing methods and a re-evaluation of acceptable lower limit levels.

The right approach to biological safety cabinets, and collaboration between engineers and those who will operate the equipment, is crucial to preventing cell-culture contamination.

The company recently launched its OmniTop Sample Tubes adjustable volume sampling system, a single-use system that allows for exact sampling.

The new filters extend column life and reduce contamination while simplifying sample preparation.

The new chromatography columns provide enhanced separation for high order aggregates and macromolecules.

The right approach to biological safety cabinets, and collaboration between engineers and those who will operate the equipment, is crucial to preventing cell-culture contamination.

Light obscuration testing is the preferred method of sub-visible particle quantification but is not suitable for every preparation.

Discovery of carcinogenic nitrosamines in three of the world’s most widely prescribed drugs is driving efforts to better detect, control and prevent their generation in APIs and finished drug products.

Solentim and ATUM will bundle together the Leap-In Transposase platform with the VIPS single cell cloning instrument for cell line development.

The new printers contain integrated PTLab software and can print directly onto slides and cassettes, eliminating handwritten mistakes and expensive labels.