
The companies will develop a software-connected pipetting system for improved reproducibility and traceability in life-science laboratories.

The companies will develop a software-connected pipetting system for improved reproducibility and traceability in life-science laboratories.

The RK-1 continuous mixing processor from Readco Kurimoto is suited for testing formulations in continuous processing conditions.

A new reactor flask heating system now offered by Asynt allows the conduction of safe experiments in academic teaching and research labs.

Spectroscopic-based control methods were introduced as equivalent alternative methods, first to a gas chromatographic method to monitor an in-process solvent exchange step and second to a potentiometric titration method to release a process input material for drug substance manufacturing.

Leveraging vast quantities of analytical data requires digitalization and platform integration.

Formulatrix’s FLO i8 and Flow Axial Seal Tip liquid handlers offer automated transferring.
Drug developers must understand the complex bioanalytical assays for cell- and gene-therapy drug development programs and ensure that partners have the specialized expertise needed for complex therapeutic classes.

Lonza’s PyroTec PRO system combines robotic liquid-handling technology with an automation software module.

The company has built a fit-for-purpose liquid chromatography–mass spectrometry (LC–MS) system to streamline analytical monitoring tests for biopharmaceuticals.

Tornado Spectral Systems, winner of the 2018 CPhI Excellence in Pharma Award for Analysis, Testing, and Quality Control, discusses real-time process measurement for biopharmaceutical and small-molecule drug manufacturing.

A new plasma B cell antibody discovery workflow launched by Berkeley Lights enables the shortening of antibody drug discovery from month to a day.

The new reagents are designed to support clinical-phase and commercialization stages of cell and gene therapy production and to enhance DNA transfection.

The Steritest NEO device from MilliporeSigma provides safer pharmaceutical product testing through additional features.

The company will use GE Healthcare’s off-the-shelf KUBio biologics factory, which is expected to start operations in 2020, to provide development and manufacturing for early- to late-clinical and early-commercial manufacturing stages.
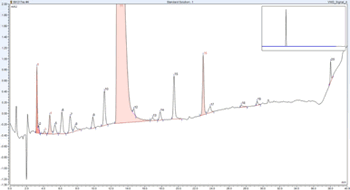
Integration of two separate chromatography data systems boosts workflow efficiency.

Charles River Laboratories has announced a data expansion in its compendium of tumor models for oncology drug R&D.

A new, high-throughput microplate reader cuts down on screening time and works faster than standard ultra-high-performance liquid chromatography processes.

The partnership, co-funded by Enterprise Ireland, will develop technologies for monitoring the quality of biopharma processes.

The contract research organization has increased its US-based early phase clinical capacity and doubled its specialty lab space.

Industry–academia collaborations seek to address unmet needs in measurement science.

Collaborative robots work beside laboratory employees to improve efficiency in pharmaceutical research and quality control labs.

The testing of raw materials is essential as raw material quality determines the outcome of biologic product quality.
By adapting techniques from other sciences-and exploring better tools for biologics drug development-researchers are addressing challenges of protein characterization.

If a vortex mixer produces too much energy during sample preparation for particle size analysis, the size and morphology of particles can change. A study compares the applied shear to sample suspensions of ibuprofen to observe the effects of applied shear on the particle size distribution.

As biopharma companies rapidly change their focus, they may lack the laboratory space, instrumentation, and the scientific knowledge to support biologics research.