
The ROSS X-Series Inline Ultra-High Shear Mixer produces quality dispersions, suspensions, and emulsions in a variety of industries.

The ROSS X-Series Inline Ultra-High Shear Mixer produces quality dispersions, suspensions, and emulsions in a variety of industries.
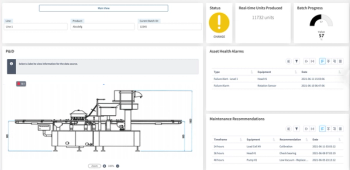
Aizon's Asset Health Application system is customized to manage each manufacturer's unique asset maintenance needs.

Linkam Scientific’s RHGen Relative Humidity (RH) controller allows for precise control of sample humidity without need for an external dry air supply.
New England Biolabs Luna Probe One-Step RT-qPCR Mix with UDG enables sensitive, linear, real-time detection of target RNA sequences.

Reducing US dependency on overseas pharma manufacturing may prove critical in navigating supply chain disruptions.

The acquisition and analysis of MS data can be made more efficient with the integration of modern software tools.

Although the bio/pharma supply chain has vulnerabilities that still need to be addressed, the COVID-19 pandemic has left lasting effects—some of them for the better.

Pfizer and BioNTech receive positive opinion for COVID-19 vaccine booster in adolescents 12 through 17 years of age in the EU.

Eli Lilly and Company are investing $700 million into a Boston-based facility for the newly announced Institute for Genetic Medicine.

Scottish Enterprise has awarded £20 million (US$27 million) to Valneva Scotland to advance vaccine development.

Alvea has begun preclinical testing of a scalable, shelf-stable DNA vaccine against SARS-CoV-2 variants.

Moderna and Thermo Fisher Scientific have formed a collaboration to leverage dedicated commercial fill/finish manufacturing capacity in the US for mRNA vaccines and therapies.

Hoffman Neopac has signed a partnership with Saperatec to begin recycling aluminum laminate composites upon its opening in mid-2023.

Quotient Sciences announces that it has integrated drug substance into its flagship Translational Pharmaceutics platform.

Recipharm has strengthened its biologics manufacturing capabilities with the acquisition of both Vibalogics and Arranta Bio.

Health Canada authorizes Novavax COVID-19 vaccine for individuals 18 years of age and older.

Thermo Fisher Scientific is expanding its Millersburg, Penn., site with a $40 million investment to support increased production of single-use technology for critical vaccines and biologics.

The commercialization and licensing deal will see Biogen commercialize Xbrane’s Xcimzane, a proposed biosimilar for CIMZIA (certolizumab pegol).

ImmunoGen and Eli Lilly and Company have entered into an agreement that gives Lilly exclusive rights to research, develop, and commercialize ADCs designed for targets selected by Lilly from ImmunoGen’s camptothecin technology.

Thermo Fisher Scientific's solution for cell and gene therapies involves a combination of cold chain logistics, serialization compliance, and distribution

In this episode of the Drug Solutions Podcast, Chris Spivey, editorial director, and Meg Rivers, senior editor, interview Sean Tucker, PhD, chief scientific officer of Vaxart, on developing oral recombinant vaccines.

Digital technology streamlines the line changeover process.

Piramal Pharma Solutions plans to construct a multipurpose ADC manufacturing and aseptic fill/finish facility in Grangemouth, Scotland, and to upgrades its existing API manufacturing facility in Morpeth, England.

China-based CDMO Asymchem acquires US-based chemical technology company Snapdragon.

In an agreement with MaaT Pharma, Skyepharma will build MaaT a dedicated 1500-m² microbiome ecosystem therapies manufacturing site.