
The company announced plans to construct a 1.3 million-ft2 integrated manufacturing center in Chengdu, China.

The company announced plans to construct a 1.3 million-ft2 integrated manufacturing center in Chengdu, China.

The acquisition of GlaxoSmithKline’s manufacturing site in Cork, Ireland, will expand Thermo Fisher Scientific’s global footprint for complex API manufacturing.

A CPhI Middle East & Africa (MEA) expert has forecasted a boom in manufacturing in the region and has expectations that within five years some of the industry will be acting as contract manufacturers for the European market.

The partnership will focus on the commercialization of inducible promoters to improve biomanufacturing.

StarTab from Colorcon was designed to ensure functionality and stability for direct compression tableting.

GE Healthcare launches Chronicle GMP-compliant automation software for cell therapy manufacturing.
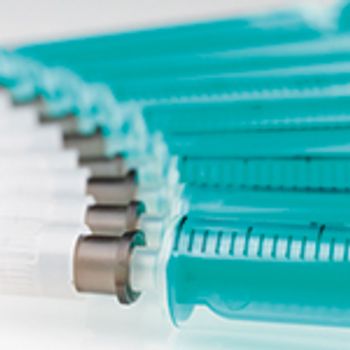
Empty and prefilled syringes must pass a range of quality control tests.

Quality cannot be verified through testing, especially at the limit of detection, and no test method can confirm the absence of a microbe or particle.

The highly customized nature of cell and gene therapy production means that manufacturing innovations for one therapy may not be easily transferable to others.

The new 40,000-ft2 facility will support Bayer's growing biologics portfolio in oncology, cardiology, and additional therapeutic areas.

Harvard University researchers used single-cell sequencing to identify a protein expressed uniquely by insulin-producing beta cells created from stem cells in the laboratory.

INTERPHEX 2019 presented solutions for ophthalmic containers, materials, and equipment for forming primary containers, child-resistant and tamper-evident (TE) cartons, and cold-chain packaging.

It has been confirmed that both Bulgaria and Cyprus will now be able to perform GMP inspections at a level considered equivalent to the United States

Vibalogics has increased its single-use bioreactor and purification capacity with a new manufacturing for its specialist oncolytic virus and viral vector manufacturing services as a result of a growth in demand.

Dyadic International has announced its collaboration agreement with Serum Institute of India for the development and manufacture of up to 12 antibodies and vaccines using Dyadic’s C1 gene expression system.

The Allen-Bradley ControlLogix 5580 controller from Rockwell Automation is compliant with the IEC 62443-4-2 security standard.

Recipharm Inhalation Solutions is an integrated service for inhalation products.

The FactoryTalk Analytics LogixAI module from Rockwell Automation detects production anomalies.

A solar panel installation at Novo Nordisk’s North Carolina facility was initiated in March 2019 as part of the company’s commitment to zero environmental impact globally.

Automated candle and pressure-plate filtration equipment for removing catalyst residues from API slurries are operated in a closed system.

Managing and controlling e-records is vital for maintaining CGMP data integrity.

The Vaccine Development and Bioprocess Cell Culture Technology Day will take place on May 16, 2019 in Baltimore, MD.

In collaboration with Pall Corportion, biotech company Freeline completed the first full-scale run at its newly commissioned, GMP gene-therapy manufacturing facility in the UK.

A data exchange program between GE Healthcare and Amgen aims to improve biologics manufacturing through analysis of how raw materials affect the process.

Closed systems for aseptic fill and finish were featured at INTERPHEX 2019.