
The company will invest an additional $60 million to expand its manufacturing facility in Durham County, NC.

The company will invest an additional $60 million to expand its manufacturing facility in Durham County, NC.

Ireland’s National Institute for Bioprocessing Research and Training received funding from the Science Foundation Ireland’s Technology Innovation Development Award program for monoclonal antibody research.

The “hands-on” seminar will be held June 12–13, 2019 and will feature educational sessions and demonstrations in the company’s process laboratory.

The partnership will expand Lonza’s offering of hematopoietic cell lines.

The Society of Plastics Engineers (SPE) Extrusion Division will host the SPE Minitec-PLUS polyethylene terephthalate (PET)/polylactic acid (PLA) Extrusion, a two-day event at the Polymers Center in Charlotte, NC, on May 1–2, 2019.

GS1, a global supply-chain standards organization, launched a new messaging standard in collaboration with GS1 US to help meet the requirements of the US Drug Supply Chain Security Act (DSCSA) for salable returns of serialized prescription drugs.

In a partnership with Thermo Fisher Scientific Pharma Services, Pacira added a manufacturing suite in Swindon, UK, that doubles the company’s capacity by mirroring the company’s facility in San Diego, CA.

The companies will jointly develop and commercialize an investigational bifunctional fusion protein immunotherapy currently in clinical development for cancer treatment.

Supply chain players are eligible to participate in FDA’s pilot program to develop an electronic system for tracking drug products.

Honeywell introduces Aclar Accel barrier film with faster production and delivery for pharma packaging.

The agency is requiring companies that make sartan blood pressure drugs to review their manufacturing processes due to the problem with impurities.

PCI Pharma Services expanded capacity of its commercial packaging site in Illinois.

In a deal potentially worth up to $460 million, Genentech and Xencor will develop and commercialize novel cytokine therapeutics.

The RK-1 continuous mixing processor from Readco Kurimoto is suited for testing formulations in continuous processing conditions.

Spectroscopic-based control methods were introduced as equivalent alternative methods, first to a gas chromatographic method to monitor an in-process solvent exchange step and second to a potentiometric titration method to release a process input material for drug substance manufacturing.

Storage and retrieval methods and the unique requirements found in building codes are crucial considerations.
Using a QbD approach in the development and formulation of topical products will enable the drug developer to provide a robust control strategy for manufacturing.

The Flexicon FPC60 peristaltic fill/finish system from Watson-Marlow Fluid Technology Group can use a variety of modules that allow users to create their own customized filling solution to suit small-batch applications.

Robotic isolators and single-use technologies are gaining ground, according to aseptic processing consultant Jim Agalloco.

Technical advances in process understanding and control must be accompanied by a change in mindset.

ChargePont Technology has revealed that it is now able to expand its smart monitoring technology into North America thanks to being granted HazLoc (Hazardous Area) certification.

The acquisition will boost Hitachi Chemical’s presence in the European regenerative medicines market.

Janssen and MeiraGTx will collaborate on the development and commercialization of gene therapies for treating inherited retinal diseases.

Tech transfer is key to succession advancing biopharmaceutical pipeline products from research to preclinical.
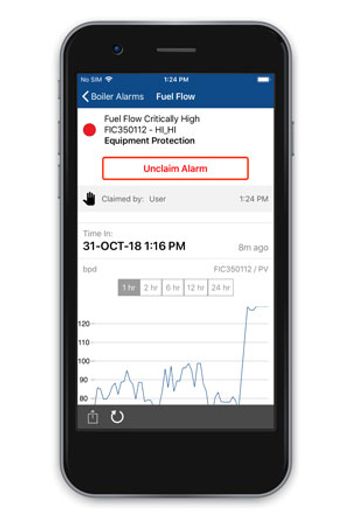
New alert customization and expanded Open Platform Communication support in Emerson’s DeltaV Mobile app improve notification and third-party system monitoring.