
Under an expanded agreement with J&J, Catalent Biologics will increase manufacturing capacity at its Anagni, Italy, site to accommodate the large-scale commercial supply of Janssen’s COVID-19 vaccine.

Under an expanded agreement with J&J, Catalent Biologics will increase manufacturing capacity at its Anagni, Italy, site to accommodate the large-scale commercial supply of Janssen’s COVID-19 vaccine.

Smart packaging opens doors to new products for diagnosis and treatment of eye problems.

TurboFil’s CrimpTech Series includes semi- and fully automatic machines.

Manufacturing of the vaccine will take place at Baxter’s fill/finish sterile manufacturing facilities located in Bloomington, IN.

Labeling, printing, and on-dose tagging technologies provide a multi-layered approach to anticounterfeiting for pharmaceuticals.

The study found that more than 90% of packages and cases scanned at three companies met all of the labeling requirements, with three years remaining until the final DSCSA deadline in 2023.

Anticounterfeiting technology protects the supply chain from manufacturer to patient.

Packaging and transporting large quantities of COVID-19 vaccines pose challenges for the cold chain.

Acorda’s Chelsea, MA facility will serve as a global center of excellence in the Catalent network for spray-dried dispersion and dry powder encapsulation and packaging.

Pharmaceutical Technology interviewed Cloudleaf about trends in tracking vaccines through the supply chain.

The films are now available in three options of varying thickness for added protection against moisture and oxygen.

Due to a packaging error, the company is voluntarily recalling one lot of sildenafil 100 mg tablets for the treatment of erectile dysfunction and one lot of trazodone 100mg tablets for the treatment of major depressive disorder.

Freezers, shipping containers, and tracking systems aid distribution of COVID-19 vaccines.

Flexible packaging benefits align with challenges including an aging population, compliance requirements, e-commerce, serialization, and a need for recyclability.

Software automates the labeling process, ensures compliance with regulations, prevents errors, and cuts costs.

Through the acquisition, PTI will gain access to LDA’s lead product, the SIMS 1915+, a helium leak test system that combines its helium leak detection technology with its SIMS software for data collection and analysis.

Sterilization of primary packaging is important for vaccines.
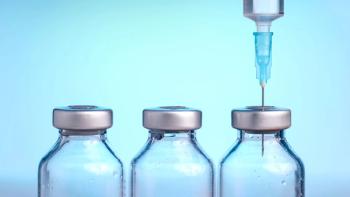
Packaging suppliers prepare to meet the demand unleashed by COVID-19 vaccines and treatments.

Eisai has announced an expansion of its manufacturing operations at its Hatfield production plant in the United Kingdom to meet global demand.

Experts fear confusion over allocation processes, cold-chain requirements, and IT systems needed to bring a COVID-19 vaccine to market.

The companies will work together to produce a wearable device that can subcutaneously deliver ketamine therapy as a non-opioid pain treatment.

The rebranding stems from the company’s expanded services which include 3PL services, supply chain transparency solutions, and retail pharma product procurement for over-the-counter medications.

Broader application of RFID in pharmaceutical packaging benefits caregivers and patients.

Polymeric containers offer an alternative to glass.

Tjoapack is investing in fully automated lines, including a PFS line with an initial capacity of 7 million syringes per year and a vial line with an eventual capacity of more than 12 million vials per year.